Unit 8.1 The Mole
8.1 The Mole and Mole
Just like how you would call 12 eggs a "dozen" eggs, the mole is used in a similar manner in chemistry. Having a mole of something means that you have 6.022 x 10^23 (Avogadro’s number) of it, so this unit is used for very small entities.
To start of, the formula for calculating the moles in an element or compound is:
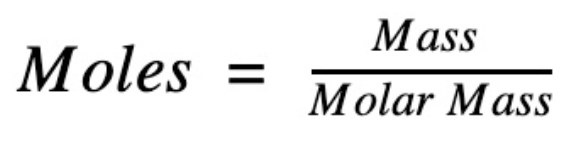
But first we need to know what molar mass is
Molar Mass
To find the molar mass of the atom Nitrogen, you would first need to look at the periodic table. You would see the atomic number 7, and then the average mass of 14.01, which would be the molar mass(14.01 g/mol) 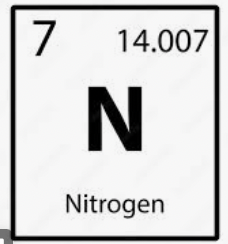
For other molecules such as O3(ozone), we can simply just multiple the atomic mass of oxygen and multiply it by three. So the molar mass of ozone is 48 g/mol.
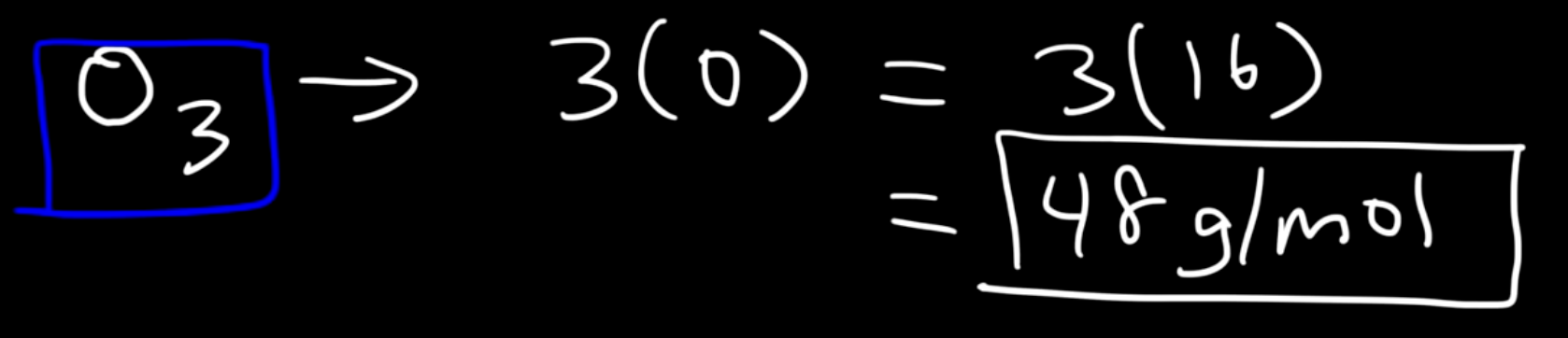
For compounds such as CO2, we can find the atomic mass of each individual element, and add them up. Carbon has an atomic mass of 12.01, while Oxygen has the atomic mass of 16. Though since there are two oxygen atoms, we multiply 16 by 2. 12.01+32 is 44.01 g/mol.
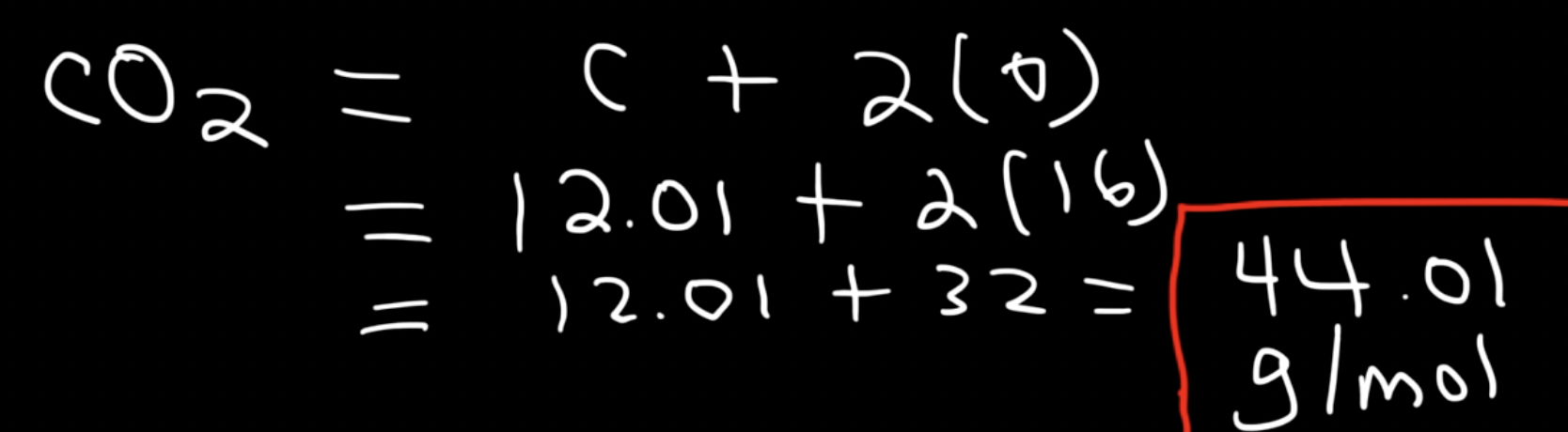
Continuing with calculating the moles
The basic formula for calculating moles is
<<#moles = mass/molar mass<<
Let’s say we wanted to know how many moles were in 230.1g of cobalt (III) fluoride.
Using the formula stated above, we would only have to find the molar mass, as the mass is already provided. Cobalt (III) fluoride’s formula is CoF3, and so we figure this out using the atomic masses on the periodic table. Next, we divide the mass by the molar mass.
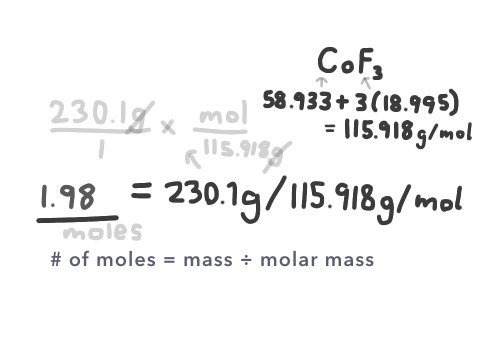
To find the atoms/molecules, you take the number of moles and multiply that by Avagadro’s number.
Step by step:
- Determine the mass of the element in grams.
- Find the atomic mass of the element from the periodic table.
- Divide the mass of the element by its atomic mass to get the number of moles.
- Multiply the number of moles by Avogadro's number, which is 6.022 x 10^23, to get the number of atoms or molecules.
Empirical Formula