Chapter 7- alkyl halides: nucleophilic substitution and elimination reactions (copy)
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
substitution
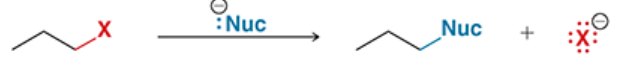
reaction in which an alkyl halide (substrate) is treated with a nucleophile
nucleophile replaces halogen
elimination reaction
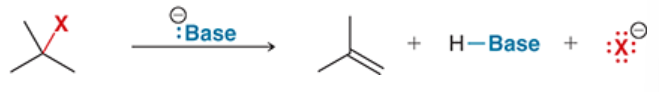
reaction in which an alkyl halide (substrate) is treated with a base
alkene (pi bond) is formed
new molecule does not have base or halogen attached
leaving group
the group separated from a compound
e.g. halogens in alkyl halides
good LGs: weak (stabilized) bases, conjugate bases of acids with pKa < 0
examples of good leaving groups
Weak bases / pka < 0
I-
Br-
Cl-
RSO3- (sulfate ions)
H2O
(note: leaving groups above are listed in order of decreasing stability, so I- is best and H2O is not as good)
OTs (excellent)
examples of bad leaving groups
pka > 0
F- (closest to good)
H-
CH3CH2O-
H2N- (worst)
SN2 reaction
reaction in which an alkyl halide is treated with a strong nucleophile
substitution reaction, involving a nucleophile (Nu), bimolecular
concerted mechanism (nucleophile attacks and LG leaves at the same time)
usually exhibits Walden inversion
rate = k[alkyl halide][nucleophile] (second order)
![<p>reaction in which an alkyl halide is treated with a strong nucleophile</p><p>substitution reaction, involving a nucleophile (Nu), bimolecular</p><p>concerted mechanism (nucleophile attacks and LG leaves at the same time)</p><p>usually exhibits Walden inversion</p><p>rate = k[alkyl halide][nucleophile] (second order)</p>](https://knowt-user-attachments.s3.amazonaws.com/53e08f5e-3983-40db-8266-c4953174f9e5.png)
rate of an SN2 reaction
rate = k[alkyl halide][nucleophile]
sensitive to number of substituents at the alpha position (degree of the alpha carbon)
methyls and primary alkyl halides are best, secondary is okay, tertiary is negligible (cannot be performed because of too much steric hindrance)
also sensitive to substituents attached to beta carbon, but not as much (same concept applies to beta carbons as to alpha carbons)
Walden inversion
in an SN2 reaction, wedges become dashes and vice versa
usually R becomes S and S becomes R (but not always)
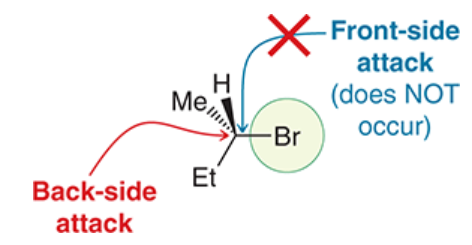
happens because of back-side attack
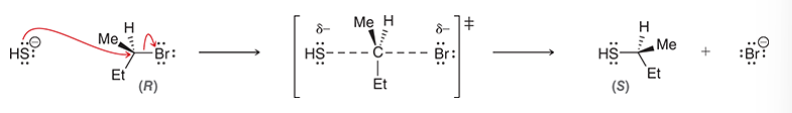
back-side attack
requirement for a Walden inversion in SN2 reaction
lone pairs of Lg create areas of high electron density that block the front side of the substrate
MO theory: electron density flows from HOMO of nucleophile to LUMO of electrophile
Nu can only attack from the back side
stereospecificity
the dependence of a product’s configuration on the starting material’s configuration
nucleophilicity
the rate at which a Nu will attack an electrophile
strong Nus give faster SN2 reactions, weak Nus give slower reactions
strong Nu is required for SN2 to be efficient
influenced by charge and polarizability
charge: negative charge makes a stronger Nu than a neutral charge (e.g. OH- vs H2O)
polarizability: ability of an atom to distribute its electron density unevenly as a result of external forces (directly related to size and number of electrons that are distant from nucleus) (e.g. halogens are strong Nus, alcohols are weak)
E2 reaction
a reaction in which an alkyl halide reacts with a strong base
elimination reaction, bimolecular
concerted mechanism
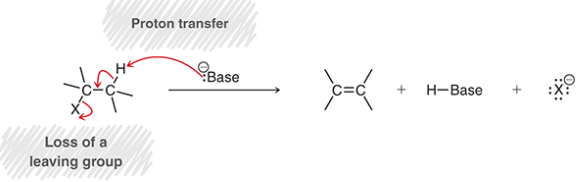
beta elimination
aka 1,2-elimination or dehydrohalogenation
a proton is removed from beta position, halide is ejected as an LG (X-) from alpha position, double bond forms between alpha and beta positions
rate of an E2 reaction
rate = k[alkyl halide][base]
any degree of alkyl halide works, because protons can be abstracted even from a bulky tertiary substrate
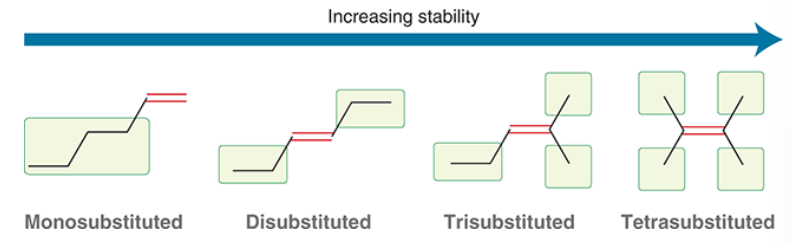
relative stability of isomeric alkenes
tetra substituted is most stable, then trisubstituted, then disubstituted, monosubstituted is least stable
because of hyperconjugation
hyperconjugation
stabilizing effect that allows alkyl groups to stabilize a carbocation by donating electron density to the neighboring sp2-hybridized C
enables delocalization of electron density
regioselectivity
a reaction that can produce two or more constitutional isomers, but produces one as the major product
occurs when beta positions of a molecule are not identical, so the double bond can form in two different regions of the molecule
regioselectivity of E2 reactions
depending on where the double bond forms and the steric bulk of the molecules, different products can be formed
Zaitsev product and Hofmann product
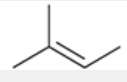
Zaitsev product
more substituted alkene
usually the major product, but not always (e.g. when both substrate and base are sterically hindered)
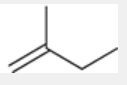
Hofmann product
less substituted alkene
e.g. formed when substrate and base are both too bulky to form the Zaitsev product
stereoselectivity of E2 reactions
trans product is major
cis product is minor
Hammond postulate: transition state for formation of trans alkene is more stable than transition state of cis alkene
stereospecificity of E2 reactions
molecule must be anti-periplanar
anti-: proton and LG must be anti on a Newman projection
periplanar: dihedral angle must be 180* (or close to 180*)
stereoselective vs stereospecific E2 reaction
stereoselective: substrate is not necessarily stereoisomeric, but substrate can produce two stereoisomeric products with one in higher yield
stereospecific: substrate is stereoisomeric, stereochemical outcome is dependent on which substrate is used
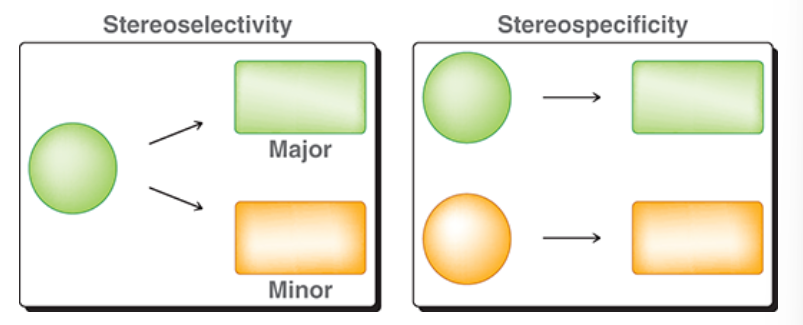
SN1 reaction
reaction in which an alkyl halide is treated with a weak nucleophile
substitution, involves a nucleophile, unimolecular
stepwise mechanism
solvolysis: solvent molecule functions as the attacking nucleophile
step 1: LG leaves, creates carbocation intermediate
step 2: Nu attack
if Nu is uncharged, step 3: proton transfer
generally not observed at sp2 hybridized centers (because loss of LG would result in an unstable carbocation)
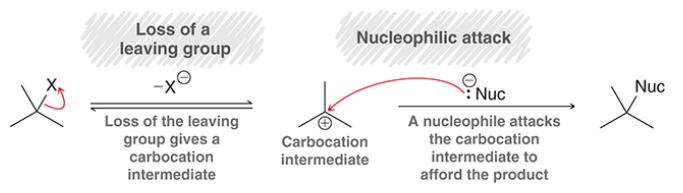
E1 mechanism
tertiary alkyl halide undergoes ionization in polar solvent, solvent acts as base and deprotonates intermediate carbocation
elimination, unimolecular
stepwise mechanism
step 1: LG leaves
step 2: proton transfer, electrons move to create double bond
generally not observed at sp2 hybridized centers (because loss of LG would result in an unstable carbocation)
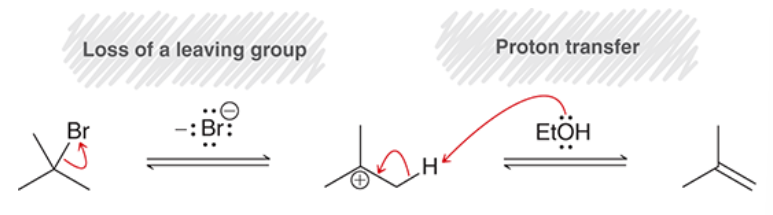
rearrangements for SN1 and E1
hydride shift and methyl shift
occur to create a more stable tertiary carbocation
(note: a methyl shift may be drawn at the same time as everything else in an SN1 process, but it is still SN1 and not concerted)
stereochemistry of SN1 reactions
retention of configuration: intermediate carbocation can be attacked from either side; does not require backside attack, so no Walden inversion
however, Nu will attack more often on the side opposite the LG, so there is slight preference to inversion over retention
predicting products: substitution vs elimination
step 1: determine function of reagent (substitution when reagent functions as Nu, elimination when reagent functions as base)
step 2: determine expected mechanism(s) based on substrate degree and reagent identity
step 3: consider regiochemical and stereochemical outcomes
reagent and substrate combinations for SN2
1* substrate and strong B/strong Nu (most)
1* substrate and weak B/strong Nu
2* substrate and strong B/strong Nu (some)
2* substrate and weak B/strong Nu
reagent and substrate combinations for E2
1* substrate and strong B/weak Nu
1* substrate and strong B/strong B (some)
2* substrate and strong B/weak Nu
2* substrate and strong B/strong Nu (most)
3* substrate and strong B/weak Nu
3* substrate and strong B/strong Nu
reagent and substrate combinations for SN1
3* substrate and weak B/strong Nu
3* substrate and weak B/weak Nu (about half)
reagent and substrate combinations for E1
3* substrate and weak B/weak Nu (about half)
regiochemical and stereochemical outcomes for SN2
regiochemical: Nu attacks alpha position, where LG is connected
stereochemical: Nu replaces LG with inversion of configuration
regiochemical and stereochemical outcomes for E2
regiochemical: Zaitsev product is generally favored over Hofmann, unless sterically hindered base is used
stereochemical: stereoselective; trans will be favored over cis when possible. Stereospecific; when beta position of substrate has 1 proton, anti-periplanar alkene will be obtained
regiochemical and stereochemical outcomes for SN1
regiochemical: Nu attacks carbocation which is generally where LG was connected (unless hydride shift or methyl shift occurred)
stereochemical: Nu replaces LG to give nearly racemic mixture, with slight preference for inversion over retained configuration
regiochemical and stereochemical outcomes for E1
regiochemical: Zaitsev product always favored over Hofmann
stereochemical: stereoselective; when applicable, trans alkene will be favored over cis
other substrates for substitution and elimination reactions
tosylates: type of sulfonate ion, one of the weakest known bases
alcohols: do not undergo SN2 reactions when treated with strong Nu, but can undergo substitution reactions under strong acidic conditions (become good LG when protonated)
solvent effects in SN2 reactions
Nu and LG are generally ionic, polar solvent needed to solvate them
polar solvents also help stabilize transition state
2 types of polar solvents: protic (H connected directly to electronegative atom) and aprotic (Hs are not connected to electronegative atom)
reactions occur much faster in polar aprotic solvents compared to protic (e.g. DMF)
reason: protic solvents have electronegative atoms with lone pairs and can form H-bonds with halogen, stabilizing them
aprotic solvents can only stabilize cations, not anions
solvent effects in SN1 reactions
polar protic solvents are more suitable
reason: protic solvents stabilize ionic intermediates and transition states (give smaller Ea)