Carbon Nanoparticles - Chapter 5 Review
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
4 Terms
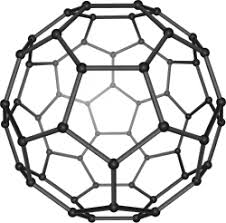
Buckminster Fullerenes
C60
Shape of soccer ball with hexagonal/pentagonal sides
Properties:
Low sublimation point (weak Van Der Waal forces, no continuous giant lattice structure)
Soft (weak IMF)
Poor Conductor (Extent of delocalized electrons like with Graphite’s p-orbitals are shorter)
Slightly Soluble
Very reactive (High electron density)
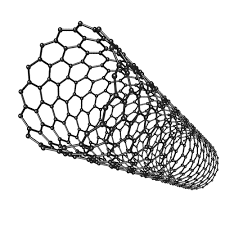
Fullerenes
Allotropes of carbon in the form of hollow spheres/tubes
Nanotubes
Fullerenes of hexagonal atoms in a cylinder
Properties:
Highly conductive (along the long axis some delocalized electrons can run across the tube)
High Tensile Strength
High Melting Point (Strong covalent bonds)
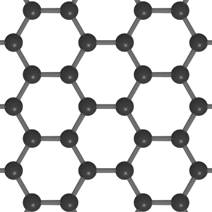
Graphene
Single layer of graphite
Properties:
Flexible
Most reactive allotrope of Carbon
Strong but lightweight
More conductive than graphite in terms of volume