Toxicology Concepts (Lecture derma, neuro, endocrine, heavy metals, heamo)
1/137
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
138 Terms
What are oligodendrocytes?
a type of glial cell found in the central nervous system (CNS)
main function is to produce the myelin sheath, a fatty layer that wraps around the axons of neurons.
What are ependymal cells?
Specialized ependymal cells in structures called the choroid plexus secrete CSF, which cushions the brain and spinal cord and helps maintain a stable environment
What do Schwann and satellite cells do?
Each Schwann cell wraps around a single segment of one axon, forming the myelin sheath.
Regulate the chemical environment around neurons (similar to astrocytes in the CNS).
Symptoms of neurodegenrative disorders
memory loss
cognitive impairment
neuronal inflammation
mitochondrial dysfunction
oxidative stress
neuron diagram
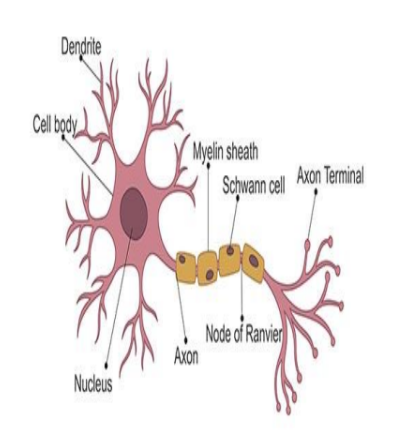
Afferent vs Efferent neurons
Afferent neurons: innervated tissue to CNS
Efferent neurons: CNS to innervated tissue
Mechanisms of protein oxidation
Carbonylation: Addition of carbonyl groups (–CO) to amino acid side chains (especially lysine, arginine, proline, threonine)
Disulfide bond formation: Between cysteine residues
Oxidation of methionine or tryptophan
Fragmentation or aggregation of proteins
6 common ROS and RNS
•superoxide (O2•−),
•hydrogen peroxide (H2O2),
•hydroxyl radical (HO•),
•hypochlorous acid (HOCl),
•nitric oxide (NO) and
•peroxynitrite (ONOO-)
What enzymes produce ROS extracellulary?
NADPH oxidases (NOX)
myeloperoxidase (MPO)
xanthine oxidase (XO)
NADPH oxidases (NOX)
Transfer electrons from NADPH to oxygen (O₂) to form superoxide (O₂•⁻)
Found in phagocyte membranes
myeloperoxidase (MPO)
Uses hydrogen peroxide (H₂O₂) to produce hypochlorous acid (HOCl)
Granules of neutrophils and monocytes.
Xanthine Oxidase
Catalyzes the oxidation of hypoxanthine to xanthine, and then xanthine to uric acid, producing superoxide (O₂•⁻) and hydrogen peroxide (H₂O₂) in the process.
Mainly in liver and vascular endothelial cells.
What does nitric oxide synthase make?
Nitric Oxide
Functions of Nitric Oxide
🫀 1. Vasodilation (Blood Vessel Relaxation)
🧠 2. Neurotransmission (Brain Signaling)
3. Immune Defense
⚠ 4. Cell Signaling and Regulation
⛔ 5. Pathological Roles
Functions of Nitric Oxide (vasodilation, neurotransmission, immune defense)
🫀 1. Vasodilation (Blood Vessel Relaxation)
It activates guanylate cyclase in smooth muscle cells → increases cGMP → causes vasodilation.
🧠 2. Neurotransmission (Brain Signaling)
It diffuses freely across membranes and modulates neural activity without needing receptors.
🧫 3. Immune Defense
Produced by inducible nitric oxide synthase (iNOS) in macrophages and other immune cells.
NO reacts with superoxide (O₂•⁻) to form peroxynitrite (ONOO⁻), a powerful molecule that helps kill bacteria, viruses, and tumor cells.
Functions of Nitric Oxide (Cell signalling, Pathological roles)
⚠ 4. Cell Signaling and Regulation
Regulates platelet aggregation (prevents unwanted blood clotting).
Influences inflammation, apoptosis, and cell proliferation.
⛔ 5. Pathological Roles
Too little NO: can contribute to hypertension, atherosclerosis, and erectile dysfunction.
Too much NO (especially from iNOS): can cause inflammation, tissue damage, and contribute to neurodegenerative diseases (e.g., Parkinson’s, Alzheimer’s).
Three types of NOS
endothelial NOS
inducible NOS
neuronal NOS
endothelial NOS
Location: Endothelial cells lining blood vessels
Type: Constitutive (always present), calcium/calmodulin-dependent
Function:
Produces low levels of NO for vasodilation
Maintains vascular tone and blood pressure
Inhibits platelet aggregation and leukocyte adhesion
Protective Role: eNOS-derived NO is anti-atherogenic (prevents atherosclerosis)
Neuronal NOS
Location: Neurons in the brain, spinal cord, and peripheral nerves
Type: Constitutive, calcium/calmodulin-dependent
Function:
Produces NO as a neurotransmitter/neuromodulator
Regulates synaptic plasticity, learning, memory, and neurovascular coupling
Also found in: Skeletal muscle, where it may regulate blood flow during muscle activity
Inducible NOS (iNOS or NOS2)
Location: Macrophages, neutrophils, and other immune or damaged cells
Type: Inducible (not normally present; expression triggered by inflammation, cytokines like TNF-α or IFN-γ)
Function:
Produces large amounts of NO as part of the immune response
NO combines with superoxide (O₂•⁻) to form peroxynitrite (ONOO⁻), which kills pathogens and tumor cells
Unregulated iNOS activity: Can cause tissue damage and contribute to chronic inflammation
diagram of oxidative stress pathway
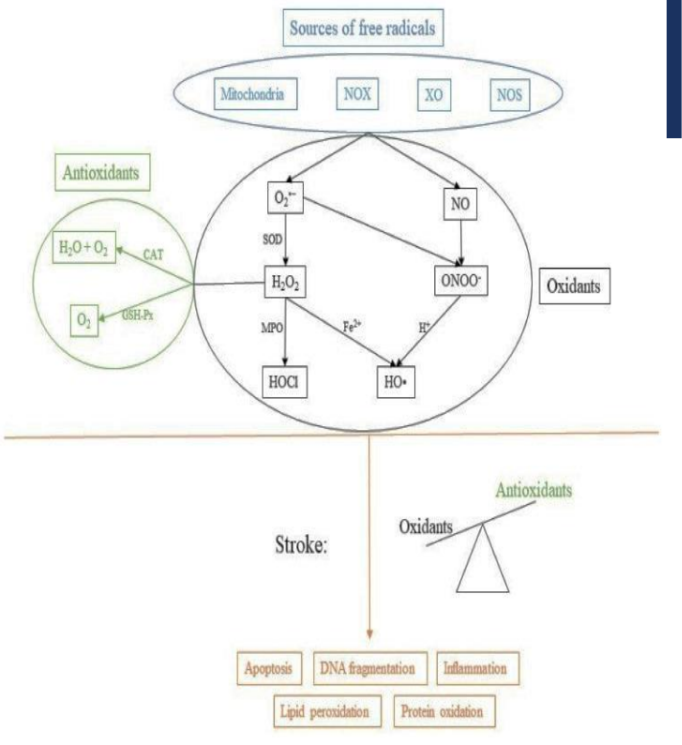
pro-oxidants
Pro-oxidants are substances that promote oxidation in cells by either generating reactive oxygen species (ROS) or inhibiting antioxidant systems.
Define lipid peroxidation
is a free radical oxidation of polyunsaturated fatty acids such as linoleic acid or arachidonic acid.
What products arise from lipid peroxidation?
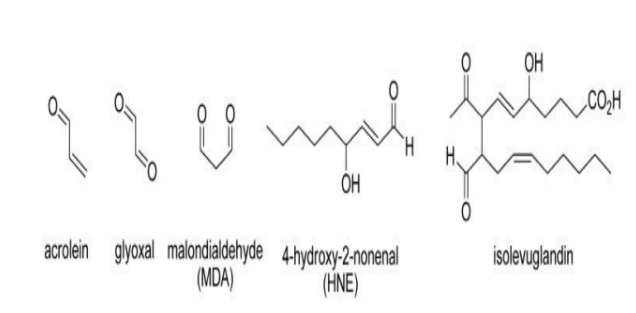
How do reactive aldehydes hurt us?
1. Protein modification
Aldehydes form covalent adducts with amino acid side chains (especially cysteine, histidine, lysine).
This changes protein structure, function, and stability.
Leads to loss of enzyme activity, misfolding, or aggregation (linked to diseases like Alzheimer’s).
2. DNA damage
Reactive aldehydes can form DNA adducts → base modifications or mutations.
MDA and acrolein can cause frameshifts, cross-links, or strand breaks.
May contribute to cancer initiation and progression.
3. Membrane damage
Lipid aldehydes further disrupt membrane integrity, increasing permeability and ion imbalance.
This affects mitochondrial function, potentially triggering cell death.
Key features of Alzheimers disease
1. Amyloid-β plaques (extracellular)
Clumps of amyloid-beta (Aβ) peptides, especially Aβ42, accumulate between neurons.
These plaques disrupt cell communication and trigger inflammation.
Amyloid comes from the cleavage of amyloid precursor protein (APP).
2. Neurofibrillary tangles (intracellular)
Made of hyperphosphorylated tau protein, which normally stabilizes microtubules.
In AD, tau detaches and forms twisted tangles, leading to neuronal transport failure and cell death.
Cefepine is a _______ known to cause neurotoxicity
cephalosporin
_____ can be used for APAP overdose
Coconut oil
_____ protects against neurotoxicity and endocrine disruption caused by paracetemol
betanine
What disease is linked to organochlorines?
Pakinsons
The elasticity of the skin is due to ______ & other extracellular matrix components secreted by _______, the main cell type of the dermis.
elastin
fibroblasts
diagram of skin layers
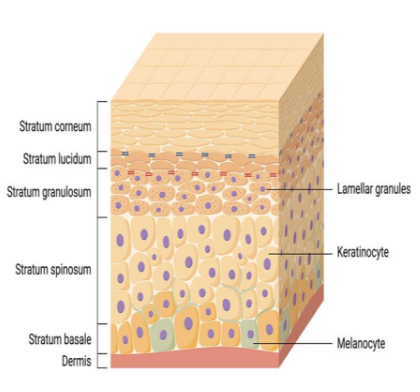
Explain the movement of keratinocytes
Keratinocytes make up ~90% of the epidermis.
They are born in the stratum basale and migrate upward through the layers, undergoing a differentiation process.
As they move up, they:
Produce keratin (a tough protein)
Lose their nucleus and organelles
Become corneocytes (dead, flattened cells)
These corneocytes are embedded in a lipid matrix (fats), forming a protective barrier against water loss, pathogens, and chemicals.
What cells are found in the epidermis?
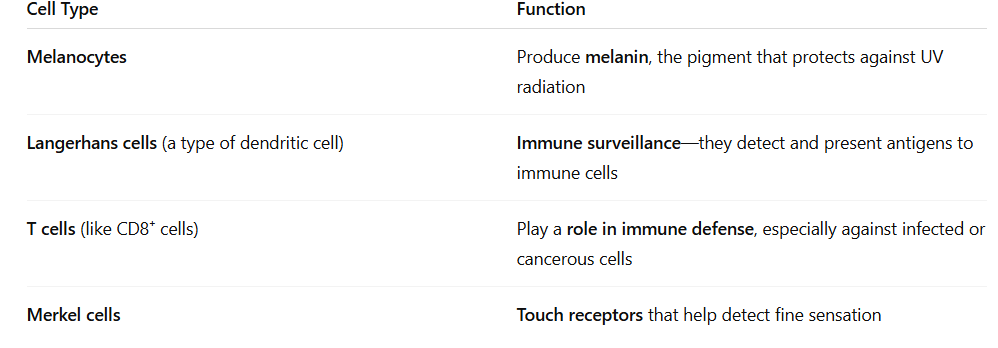
Melanocytes
protecting the skin from UV
radiation through the production
of melanosomes (melanin) that are
transferred to keratinocytes to protect their
nuclei (preventing DNA damage ).
Merkel cells:
are epithelial neuroendocrine cells. rare and are found
in the basal layer of the epidermis and around the bulge region
of hair follicles.
Dendritic cells
are specialized, migratory antigen presenting cells --- able to regulate immune
reactions. ----- sensitive to signals derived from microbes, allergens, and the airway tissue
microenvironment. central role in the establishment of long-lasting adaptive immunity
Types of cutaenous toxicity
Contact dermatitis. ...
• Photosensitive dermatitis. ...
• Chemical-induced acne. ...
• Pigmentary disturbance. ...
• Drug rash (cutaneous reaction) ...
• Hair disturbance. ...
• Nail disturbance.
Contact Dermitis
Contact Dermatitis
Cause: Direct contact with an irritant (e.g., detergent, acid) or allergen (e.g., nickel, poison ivy).
Symptoms: Redness, itching, swelling, sometimes blisters.
Types:
Irritant contact dermatitis (non-immune)
Allergic contact dermatitis (immune-mediated)
Photosensitive dermatitis
. Photosensitive Dermatitis
Cause: Exposure to sunlight + certain drugs or chemicals (e.g., tetracyclines, perfumes).
Symptoms: Redness, itching, rash in sun-exposed areas.
Types:
Phototoxic: direct damage from sunlight + substance.
Photoallergic: immune reaction triggered by sun + substance.
Chemical-induced acne
3. Chemical-Induced Acne
Cause: Long-term exposure to oils, tar, chlorinated compounds.
Symptoms: Acne-like eruptions (comedones, papules).
Example: "Chloracne" from industrial chemicals like dioxins
Pigmentary Disturbance
🎨 4. Pigmentary Disturbance
Cause: Drugs or chemicals that affect melanocytes or pigment production.
Types:
Hyperpigmentation: e.g., from minocycline, antimalarials
Hypopigmentation: e.g., from phenol or corticosteroids
Drug Rash
💊 5. Drug Rash (Cutaneous Drug Reaction)
Cause: Allergic or toxic response to medications.
Symptoms: Range from mild rashes to severe (e.g., Stevens-Johnson syndrome).
Common culprits: antibiotics, NSAIDs, anticonvulsants.
Hair disturbance
💇♂ 6. Hair Disturbance
Cause: Medications, hormones, or toxic chemicals.
Types:
Hair loss (alopecia): e.g., chemotherapy, retinoids
Excess hair growth (hypertrichosis): e.g., steroids, minoxidil
Nail disturbance
💅 7. Nail Disturbance
Cause: Drugs, systemic illness, or toxic exposure.
Examples:
Beau’s lines: grooves across nails (seen after chemotherapy)
Onycholysis: nail separates from bed (e.g., from tetracycline + sun)
Effects of pollutants on skin (melanin/sebum)
1. Melanin and Sebum Modifications
Melanin: The skin may produce more melanin in response to pollutants and UV (causing pigmentation irregularities or dark spots).
Sebum: Pollutants can oxidize sebum (the skin's natural oil), leading to inflammation, clogged pores, or acne-like symptoms.
Effects of pollutants on skin (skin erythmea)
🌡 2. Skin Erythema
Erythema = abnormal redness of the skin, due to increased blood flow in response to irritation or inflammation.
UV + pollutants trigger inflammatory responses, causing visible redness and sensitivity.
Effects of pollutants on skin (trans epidermal water loss)
💧 3. Increased Trans-Epidermal Water Loss (TEWL)
TEWL is the loss of water through the skin, normally controlled by the skin barrier.
Pollutants weaken the skin barrier, allowing more water to escape, leading to dryness, dehydration, and rough texture.
Polycyclic hydrocarbons are linked to_____
inflammatry skin disease
SDBS caused damage to ______
gills, skin, and blood of fish
Heavy metals
A metal having an atomic weight greater
than sodium, a density greater than 5 g/cm3
Arsenic 5.7; cadmium 8.65; lead 11.34;
Properties of heavy metals
High Density and Atomic Weight
Metallic Luster and Malleability
Variable Oxidation States
Low Reactivity (in some cases)
Toxic exposure to metals and metallic elements depends on
The type of exposure (inhalation, dermal absorption, or ingestion)
The species (salt, element, vapor)
Dose and duration.
Host-based factors that can impact metal toxicity include (age at exposure, gender, and capacity for biotransformation)
Young: sensitive, consume more food, higher absorption in GI, rapid growth
Lifestyle factors such as smoking or alcohol ingestion may have direct or indirect impacts on the level of metal intoxication.
How are metals naturally distributed?
Rainwater dissolves rocks and transports materials, including metals, to rivers & underground water (e.g, arsenic), depositing and stripping materials from adjacent soil and transporting these substances to the ocean to b precipitated as sediment or taken up into forming rainwater to be relocated elsewhere
Overview of metal toxicity
What does absorption of heavy metals depend on?
Solubility of metal in fluids of the intestinal tract
Chemical forms of metal (lipid soluble methyl mercury is completely absorbed compare to inorganic mercury –poorly absorbed)
Presence and composition of other materials in GI tract
Composition for absorption sites between similar metals (zinc & cadmium or calcium & lead)
Physiological state of the person exposed (Vitamin D enhance the absorption of lead)
Metals in blood plasma are bound to
__________ and ___________
plasma proteins and amino acids
Metals bound to low molecular weight proteins and amino acids are filtered in __________ into fluid of the renal tubule
glomerulous
Some metals (Cd & Zn) are effectively resorbed by _________ before they reach the urinary bladder where very little resorption occur
tubular epithelia
Absorbed metal may also excreted into intestinal tract in ___, ____, or _____
bile, pancreatic secretion or saliva
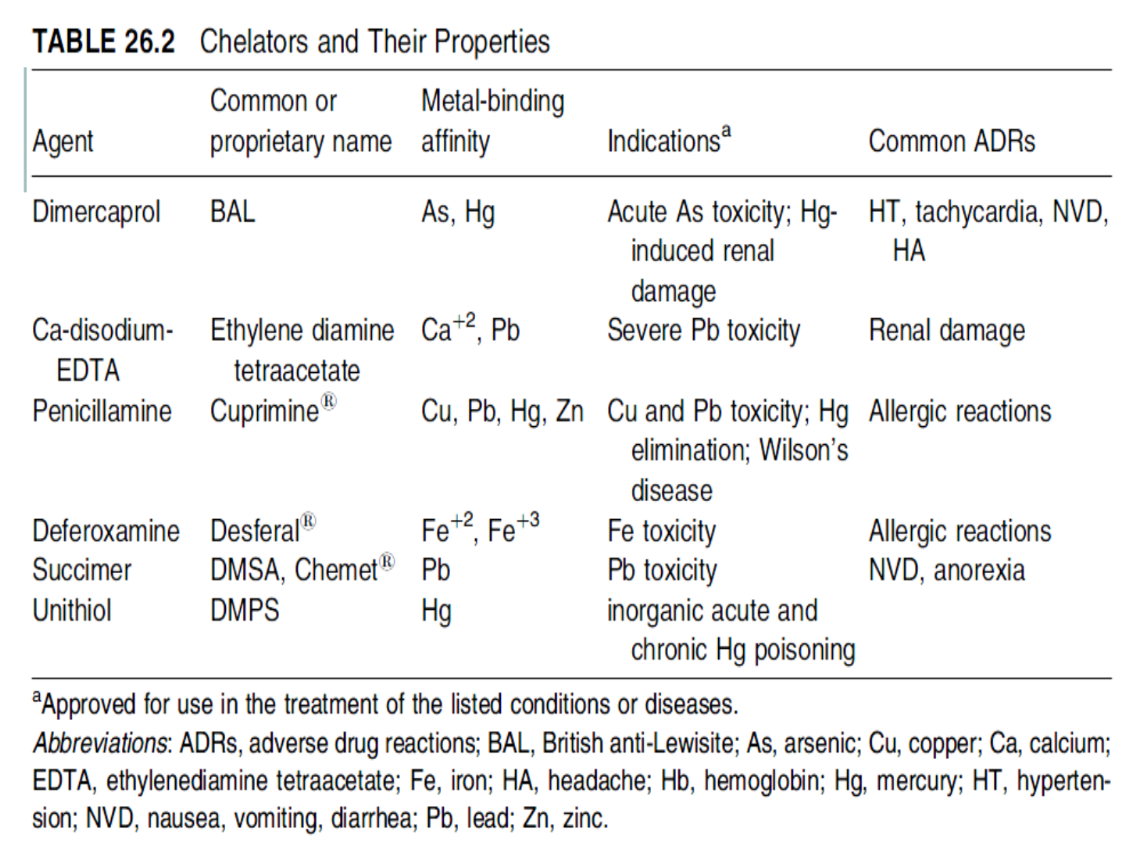
What is chelation therapy
Chelating agents (such as EDTA, dimercaprol, or DMSA) bind to metals in the bloodstream, forming stable complexes. These are then excreted via urine.
Common chelating agents
EDTA | Lead, calcium overload |
Dimercaprol (BAL) | Arsenic, mercury poisoning |
DMSA | Lead, mercury (oral form) |
Deferoxamine | Iron overload |
Requirements of being a chelator
a) Should have minimal risks involved with their therapeutic use.
b) Complex should be water soluble to enhance elimination through the kidneys without causing additional toxicity.
c) Oral administration is desirable, especially for treatment of chronic metal toxicity.
Table of chelators and their properties
Occurrence of lead
batteries and in sheathing electric cables
protective shielding from X rays and radiation from nuclear reactors
pigments in paint, until it was banned as an environmental pollutant in the United States in the 1970s.
Sources of lead exposure
Soil and dust
Paint chips
Contaminated water
Parents lead-related occupation
Folk remedies
Congenital exposure
Mechanism of lead toxicity (STUDY LATER)
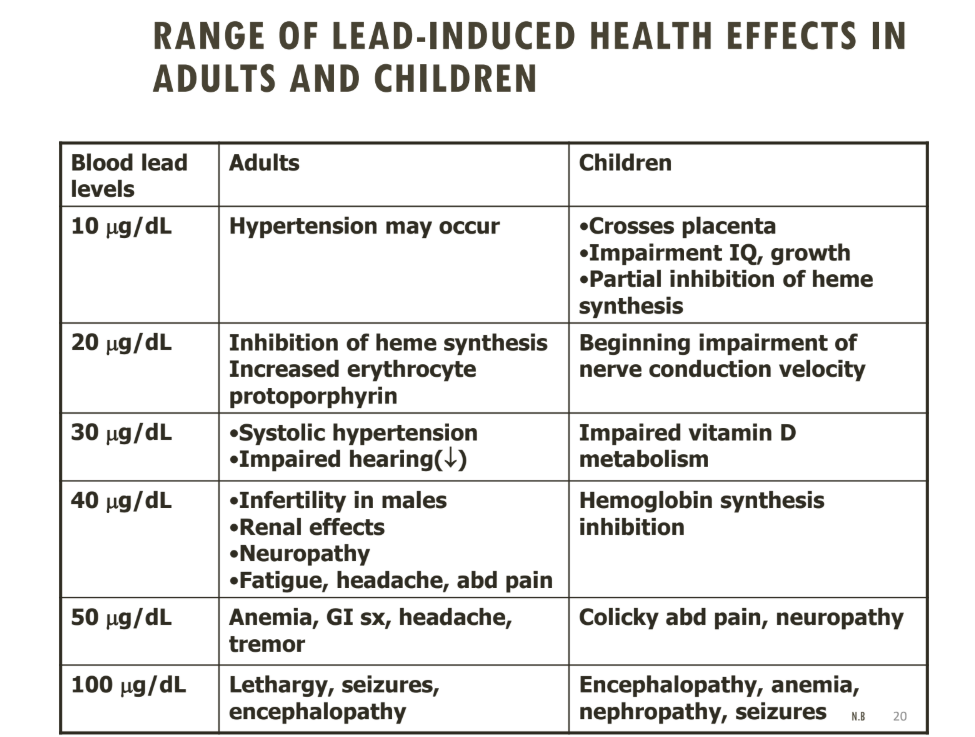
Doses of lead and effects
Diagnosis of Lead Poisoning
Evaluation of clinical symptoms and signs
CBC
Serum iron levels, TIBC, ferritin
Abdominal radiographs (for recent ingestion of lead-containing
material)
Whole blood lead level
X-ray fluorescence (XRF)- to asses body burden
APP70 increase, SOD1, prdx6 APP 695 decrease is associated with what?
lead exposure
Endocrine glands consist of:
The adenohypophysis produces and secretes a majority of the hormones of the pituitary gland
Toxicants can influence the synthesis, storage, and release of hypothalamic-releasing hormones, adenohypophyseal-releasing hormones, and the endocrine gland–specific hormones.
Corticotropin-releasing hormone (CRH) is secreted by the _________
hypothalamus
Corticotropin-releasing hormone travels through the _______________
hypophyseal portal system (a capillary and vein network linking the hypothalamus to the anterior pituitary, or adenohypophysis).
In the anterior pituitary, CRH stimulates the cleavage of __________
pro-opiomelanocortin (POMC).
POMC is a prohormone that gives rise to
Adrenocorticotropic hormone (ACTH)
Melanocyte-stimulating hormone (MSH)
Beta-endorphins
ACTH enters the bloodstream and targets the _________
It stimulates the synthesis and release of _______, a key stress hormone.
adrenal cortex
cortisol
How does cortisol regulate it’s pathway?
The hypothalamus (reducing CRH)
The anterior pituitary (reducing ACTH)
What are prohormones?
biologically inactive precursor molecules that are converted into active hormones through enzymatic processing. They are part of the body's hormone regulation system, ensuring that hormones are activated only when and where needed.
GH effects on hormones
Stimulates Protein Synthesis
Increases transcription and translation of genes involved in cell growth.
Increases Amino Acid Uptake
Enhances the transport of amino acids into cells, promoting muscle and tissue growth.
GH stimulates the GH to produce _________
somatomedins, mainly:
Somatomedin C, also known as Insulin-like Growth Factor 1 (IGF-1)
Functions of IGF-1
• Enhance T-cell proliferation
• Increase in the absorption of calcium from the intestines and decreases
the loss from urinary excretion
• Stimulates hepatic glycogenolysis to raise levels of glucose in the blood
• Increase in the growth of soft and skeletal tissues
• Increase uptake of non-esterified fatty acids by the muscle.
Function of TSH
to stimulate the thyroid gland to produce and release:
Triiodothyronine (T3)
Thyroxine (T4)
Describe the regulation of TSH secretion
Initiation:
The hypothalamus secretes Thyrotropin-Releasing Hormone (TRH).
Portal System Transport:
TRH travels through the hypophyseal portal system to the anterior pituitary (adenohypophysis).
Stimulation:
TRH stimulates thyrotrophic (thyrotrope) cells in the anterior pituitary to secrete TSH.
Thyroid Activation:
TSH travels via the bloodstream to the thyroid gland, where it promotes:
Iodine uptake
Synthesis of thyroglobulin
Release of T3 and T4
Explain the negative feedback loop of TSH
Elevated levels of T3 and T4 inhibit:
TRH release from the hypothalamus
TSH secretion from the anterior pituitary
What is an endocrine disruptor?
Mimic natural hormones (e.g., estrogen), binding to receptors and triggering inappropriate responses.
Block hormone receptors, preventing the real hormone from binding.
Alter hormone production, metabolism, or elimination, leading to hormone imbalances.
Sources of endocrine disruptors
pesticides/herbicides
personal care products
pharma drugs
industrial chemicals
What endocrine disruptors are in personal care products
Triclosan
Phthalates
DEHP Bis-2-ethylhexyphthalate
Triclosan:
where
Linked to
Antibacterial soaps
Toothpaste
Cosmetics
Toys Furniture
Endocrine disruption
Breast cancer
Resistant bacteria
Bioaccumulation
Endorcine distuptors: pesticides/herbicides/fungicides
endosulfan
DDT
Vinclosan
Endosulfan is an ________
organochlorine
what endocrine disorders are pesticides associated with?
endometriosis, early puberty, cervical cancer
What is antiandrogernic?
substance that keeps androgens (male sex hormones) from binding to proteins called androgen receptors
What is a teratogen?
any substance that can interfere with normal prenatal development, potentially causing birth defects or other abnormalities in a developing fetus
What endocrince disruptors aresynthetic/naturally occurring hormones?
phytoestrogens
diethylstilbisterol
xenoestrogens
Food with the most phytoestrogens are ______
soya, wheat, pulses
how does diethylstilbisterol disrupt endocrines
Diethylstilbestrol (DES) is a synthetic estrogen that disrupts the endocrine system by:
Mimicking estrogen – It binds strongly to estrogen receptors, overstimulating estrogenic pathways.
Altering gene expression – It causes long-lasting epigenetic changes, especially when exposure happens in the womb.
Disrupting hormone balance – It interferes with feedback loops in the reproductive hormone system.
Effects of endocrine disruptors on humans
🧠 1. Neurodevelopmental Disorders
Interference with thyroid hormones (essential for brain development)
Linked to:
Reduced IQ
Attention Deficit Hyperactivity Disorder (ADHD)
Autism Spectrum Disorders (ASD)
🧬 2. Reproductive and Developmental Effects
In females:
Early puberty (linked to BPA, phthalates)
Irregular menstrual cycles
Increased risk of polycystic ovarian syndrome (PCOS)
In males:
Lower sperm count
Testicular dysgenesis
Cryptorchidism (undescended testes)
Hypospadias (abnormal urethral opening)
🩺 3. Hormone-Related Cancers
Endocrine disruptors can mimic estrogen or testosterone, promoting tumor growth.
Breast cancer
Prostate cancer
Testicular cancer
🧠 4. Metabolic Disorders
Disruption of insulin and other metabolic hormones
Linked to:
Obesity
Type 2 diabetes
Non-alcoholic fatty liver disease (NAFLD)
🧬 5. Thyroid Dysfunction
Disruption of thyroid hormone synthesis or signaling
May cause:
Hypothyroidism
Goiter
Impaired growth and brain development in children
Why are hematopoeitic cells particular sensitive target?
cytoreductive drugs (reduce cell count):
Methothrexate, Hydroxyurea
antimitotic agents (block cell division):
Paclitaxel, Colchicine
Consequences of direct or indirect damage to blood cells
Hemolysis from toxins
infections
mechanical damage
bone marrow failure
tachycardia
Primary toxicity
Toxic effects that result directly from a substance's interaction with target cells or organs
Direct cellular or biochemical interaction (e.g., DNA damage, enzyme inhibition)
Usually rapid or predictable from known mechanism of action