Chemistry Aqueous
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms

Divide BEFORE finding the square root


When pH is decreased, include equation of H3O+ reacting with CO3- to show removal of CO3- hence equilibrium favouring forward reaction to replace it, making less solid. When execs ammonia is added Ag+ is REMOVED to form the complex ion and SHOW EQUATION for this. Formation of a complex ion INCREASES solubility as since Ag+ is removed, equilibrium favours the forward reaction to make up for it, so more solid dissolves.


When something WILL form a percipitate but we don’t know WHEN use Ks Expression using THE SOLID PERCIPITATE to calculate the conc. of Hydronium or hydroxide to find the pH.


When algebraically removing log remember that BOTH the acid and the base are multiplied by log function so keep log on both sides. THEN use inverse log to solve for conc. When volume of buffer solution is increased NOT due to water but due to SOLUTION BEING ADDED there is NO CHANGE in the pH as the ratio of the concentration of weak acid to its conjugate base REMAINS THE SAME

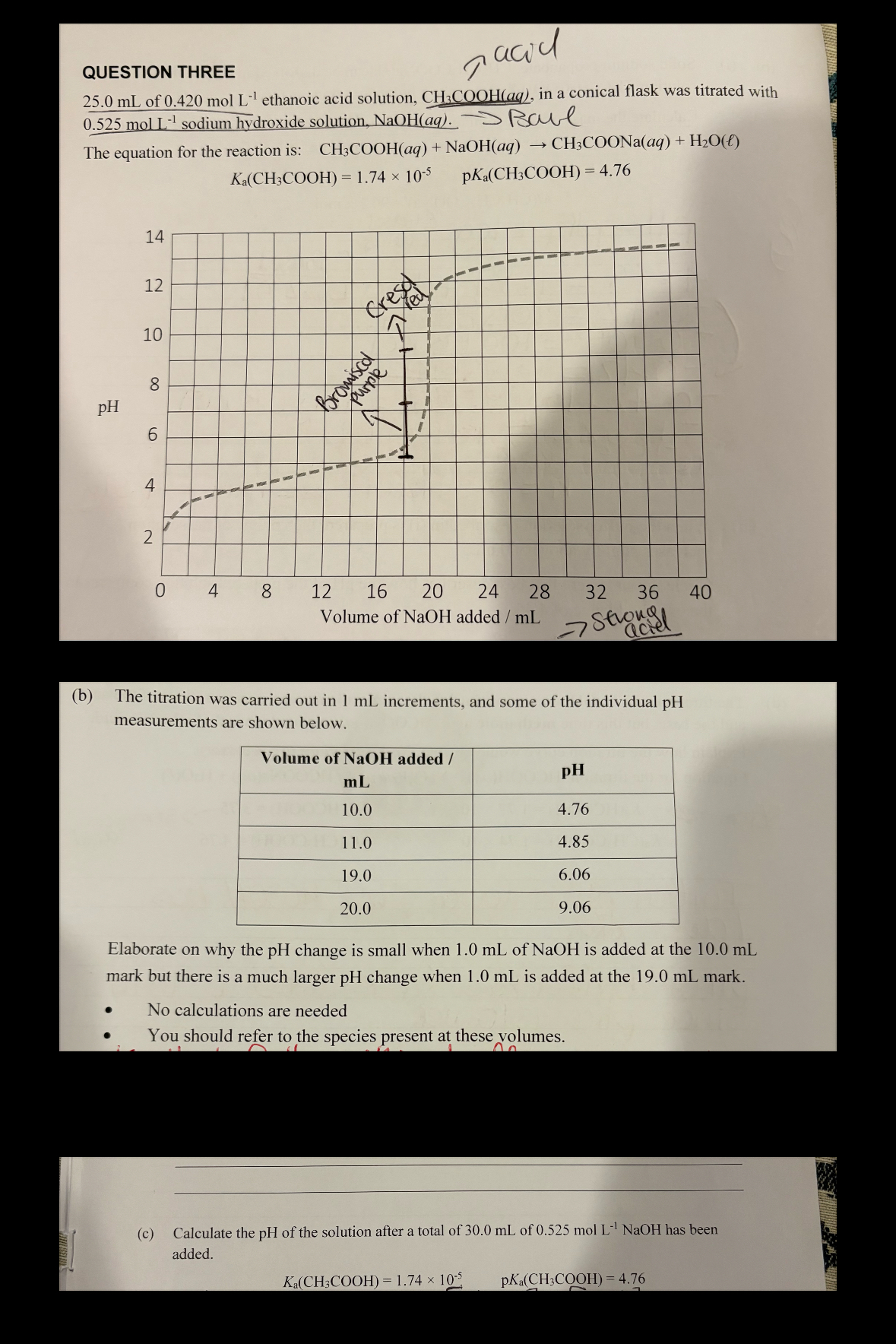
If strong acid/base is added to a BUFFER where pH IS EQUAL to the pKa, the added base is reacting with the acid present with no significant increase in pH. By the time the VERTICAL region of the curve occurs, there is almost none of the original solution left (seen here as the pH rises vertically) therefore the pH rises steeply when the strong base is added. To calculate for when strong base is present, the concentration of the OH- is equal to the concentration of the strong base. First figure out the amount from when it was unreacted (how much was added when pKa occurs) then find the concentration by dividing the amount by the OVERALL volume. Then use this to find pH.


Initial pH is lower in stronger acids and so is the buffer zone as stronger acids have lower pKa values. pH at equivalence point would also be lower as stronger acids have weaker conjugate bases and vice versa. Include buffer zone range.


When a solution contains a common ion, equilibrium favours the back reaction since there is extra F-, it will try to counter act it by using the back reaction BACK into a solid therefore the solubility will DECREASE.


Since a buffer is a solution WHICH UNDERGOES MINIMAL CHANGE IN PH WHEN SMALL AMOUNTS OF ACID OR BASE ARE ADDED, The HCOOH will react with the (Hypothetically) OH- however this produces its conjugate base AND WATER, so minimal pH change. As it is a buffer, instead of the weak acid reacting again, the conjugate base HCOO- will react with the (hypothetically) added H3O+ ions so there is almost no change in H3O+ conc as the conjugate base now goes on to produce the weak acid AND WATER.


As the [base]/[acid] in buffer equation is a RATIO, you do not need to calculate the concentration as the VOLUME REMAINS THE SAME (n=cV). To find the amount of NaOH use n=m/M then n=cV for HCOOH. you can do all this using an ICE table. Then find the amount of HCOONa and the remainder of HCOOH. This can be used to find the pH which is less than the pKa. So while it works as a buffer as it contains A WEAK ACID AND ITS CONJUGATE BASE, as the pH is lower than the pKa, it. would not be as effective at buffering added H3O+ ions as it would OH- ions.
![<p>As the [base]/[acid] in buffer equation is a RATIO, you do not need to calculate the concentration as the VOLUME REMAINS THE SAME (n=cV). To find the amount of NaOH use n=m/M then n=cV for HCOOH. you can do all this using an ICE table. Then find the amount of HCOONa and the remainder of HCOOH. This can be used to find the pH which is less than the pKa. So while it works as a buffer as it contains A WEAK ACID AND ITS CONJUGATE BASE, as the pH is lower than the pKa, it. would not be as effective at buffering added H3O+ ions as it would OH- ions. </p>](https://knowt-user-attachments.s3.amazonaws.com/628d55cc-f0c6-4e63-b4bb-ae258da25a7d.undefined)
HCl
Strong acid
HBr
Strong acid
HNO3
Strong acid
H2SO4
Strong acid
NaOH
Strong base
KOH
Strong base
Ca(OH)2
Strong base
CH3COOH
Weak acid
HCOOH
Weak acid
HOBr
Weak acid
HF
Weak acid
NH3
Weak base
CH3NH2
Weak base