chem1013-quiz 4: end of chapter 3 and chapter 4
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
What is mass percent composition?
each element’s percentage of total compound’s mass
We find the empirical and molecular formulas of a compound if what two things are known?
mass percent composition and molar mass
What is the formula of mass percent of an element?
[(mass of the element in 1 mol of compound) / (total mass of one mole of the compound)] x100
Calculate the mass percent composition of Cl in CCl2F2.
To calculate the mass percent composition of Cl in CCl2F2, divide the mass of Cl in one mole of CCl2F2 by the total mass of one mole of CCl2F2, then multiply by 100.
2(molar mass Cl)
_______________ X 100
molar mass CCl2F2
=[2(35.453 g/mol)] / [12.011 + 2(35.453) + 2(18.998)] X 100
= [70.906] / [120.911] X 100 = 58.7%
How can we use mass percent as a conversion factor?
since mass percent = per hundred, there are (mass percent in grams) per 100 grams of compound
ie: Cl is 58.7% mass of CCl2F2 → 58.7 g Cl : 100g CCl2F2
This allows us to convert between grams of an element and grams of the compound.
What do conversion factors tell us of an element in a compound?
ie: 1 mol CCl2F2 has 2 mol Cl atoms, thus, 1 mol CCl2F2 : 2 mol Cl
This means that for every mole of the compound, there are a specified number of moles of the element, allowing for stoichiometric calculations.
If you want to use the chemical formula as a conversion factor, what must you do first?
convert from grams to moles; the chemical formula indicates mol→ mol ratios, not mass;
therefore, you need to know the molar mass of the compound to make the conversion.
If we do not know the chemical formula of a compound, but know the percent mass of each element in the compound from experimental data, what can we determine?
empirical formula
What are the steps to calculate the empirical formula?
Convert the percent composition of each element to grams.
Convert grams to moles for each element.
Divide the moles of each element by the smallest number of moles to find the simplest ratio.
If necessary, multiply the ratio by a whole number to get integer subscripts.
What are the steps to calculate the molecular formula?
Determine the empirical formula.
Calculate the molar mass of the empirical formula.
Divide the molar mass of the compound by the molar mass of the empirical formula.
Multiply the subscripts in the empirical formula by this ratio to find the molecular formula.
molar mass= molar mass of empirical formula x n
find n
multiply subscripts in empirical formula by n
What is combustion analysis and it is used to find what kind of empirical formulas?
the unknown compound undergoes combustion in the presence of pure oxygen to produce carbon dioxide and water, allowing for the determination of the empirical formula by measuring the amounts of these products.
What are organic compounds?
composed of carbon, hydrogen, and a few other elements such as nitrogen, oxygen, and sulfur
What are hydrocarbons?
compounds composed of carbon and hydrogen only
What are alkanes?
single bonds
What are alkenes?
double bonds
What are alkynes?
triple bonds
All other organic compounds can be thought of as hydrocarbons with 1 or more ______— characteristic atoms or groups of atoms
functional groups
What is the prefix 1 for hydrocarbons?
meth-
What is the prefix 2 for hydrocarbons?
eth-
What is the prefix 3 for hydrocarbons?
prop-
What is the prefix 4 for hydrocarbons?
but-
What is the prefix 5 for hydrocarbons?
pent-
What is the prefix 6 for hydrocarbons?
hex-
What is the prefix 7 for hydrocarbons?
hept-
What is the prefix 8 for hydrocarbons?
oct-
What is the prefix 9 for hydrocarbons?
non-
What is the prefix 10 for hydrocarbons?
dec-
What is the affix for single bonds of hydrocarbons?
-ane
What is the affix for double bonds of hydrocarbons?
-ene
What is the affix for triple bonds of hydrocarbons?
-yne
How do you name simple, straight-chain hydrocarbons?
identifying the number of carbon atoms and adding the appropriate suffix (-ane, -ene, or -yne) based on the bonding type.
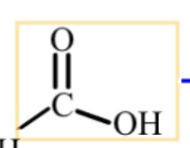
carboxylic acid

amine
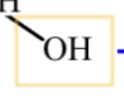
alcohol
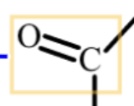
ketone
What is a chemical reaction?
1+ substances are converted to 1+ different ones, represented by a chemical equation
What must a chemical equation be, according to the law of conservation of mass?
balanced, with equal numbers of each type of atom on both sides
How are states specified using abbreviations in parentheses?
States are specified as (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous.
What can you change and what can you not change when balancing equations?
You can change the coefficients in front of compounds, but you cannot change the subscripts within a chemical formula.
What are the steps to balance chemical equations?
write a skeletal equation (unbalanced)
count # atoms on each side of the arrow
use whole number coefficients to balance
*start with the most complex compound and leave the pure for last
check for lowest whole number coefficients
What is reaction stoichiometry?
numerical relationships between reactants and products in a balanced chemical equation
What does reaction stoichiometry help us predict?
The amount of product formed from a given amount of reactant, or how much of 1 reactant is required to react with a given amount of another, in a chemical reaction.
What do coefficients specify?
relative amounts in moles of each substance