Matter Study Guide
5.0(1)
Card Sorting
1/30
Earn XP
Description and Tags
Last updated 5:21 PM on 11/17/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
1
New cards
define matter
anything that takes up space
2
New cards

does the image show solid, liquid, or gas
solid
3
New cards
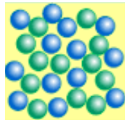
does the image show solid, liquid, or gas
liquid
4
New cards
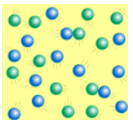
does the image show solid, liquid, or gas
gas
5
New cards
list four indicators of chemical change
bubbles (creation of gas), temperature change, color change, production of an odor
6
New cards
list four types of matter
atom/element, molecule/compound, homogeneous mixture, heterogeneous mixture
7
New cards
list four states of matter
solid, liquid, gas, plasma
8
New cards
list properties of solids
particles do not move much, definite shape(doesn't change), not much empty space
9
New cards
list properties of liquid
particles slide around, not much empty space, takes on the shape of its container
10
New cards
list properties of gas
particles move a lot, a lot of empty space, very compressible, takes on the shape of its container
11
New cards
list properties of plasma
adds heat to a gas, releases electrons, charged atoms
12
New cards
new substance means
different molecules present
13
New cards
define physical property
observation of a substance without changing it
14
New cards
list examples of physical property
smell, color, density, texture, boiling point, melting point
15
New cards
define physical change
no new substance made, same molecules present before and after, easily reversable
16
New cards
list examples of physical change
change of state, change of shape, change of size, making kool aid
17
New cards
define chemical property
ability of a substance to have a reaction
18
New cards
list examples of chemical property
ability for paper to burn in air, ability for iron to rust
19
New cards
define chemical change
a new substance is made, different molecules present after the reaction
20
New cards
list examples of chemical change
bubbles, heat released, burning, cooking,
21
New cards
define pure substances
has a set composition, same globally, cannot be broken down by physical changes
22
New cards
define atoms
smallest particles
23
New cards
define compounds/molecules
made of two or more atoms (ex: H2O)
24
New cards
define mixtures
has several pure substances mixed together, can be broken down by physical changes
25
New cards
what are the two types of mixtures
homogeneous mixture, heterogeneous mixture
26
New cards
define homogeneous mixture
consistent composition (ex: milk, lotion, salt water)
27
New cards
define heterogeneous mixture
irregular composition (ex: chocolate chip cookies, salad, granite)
28
New cards

what type of particle is this
element
29
New cards
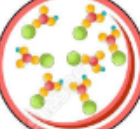
what type of particle is this
compound
30
New cards
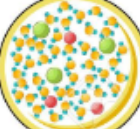
what type of particle is this
homogeneous mixture
31
New cards

what type of particle is this
heterogeneous mixture