Chemistry Chapter 5: Covalent Bonding
1/12
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Covalent Bond
Electrons are shared between two atoms nonmetal + nonmetal
VSEPR Theory
Valence shell electron pair repulsion
This determines the shape of a molecule
Nonpolar Covalent Bond
Equal sharing of electrons
Two of the same nonmetals or an H-C bond
Polar Covalent Bond
Unequal sharing of electrons
Two different nonmetals
Polar Molecule
Polar bonds and not symmetrical
OR nonpolar bonds
Dispersion forces
Attractions between nonpolar molecules. They happen when nonpolar molecules become quickly and temporarily polar. Generally they are very quick attractions. The larger the electron cloud, the stronger the attraction.
Dipole-dipole force
Attraction between polar molecules
Hydrogen bonds
Attraction between an H that is attached to an N, O, or F in one molecule to an N, O, or F in a different molecule. (Same as a dipole-dipole force but stronger)
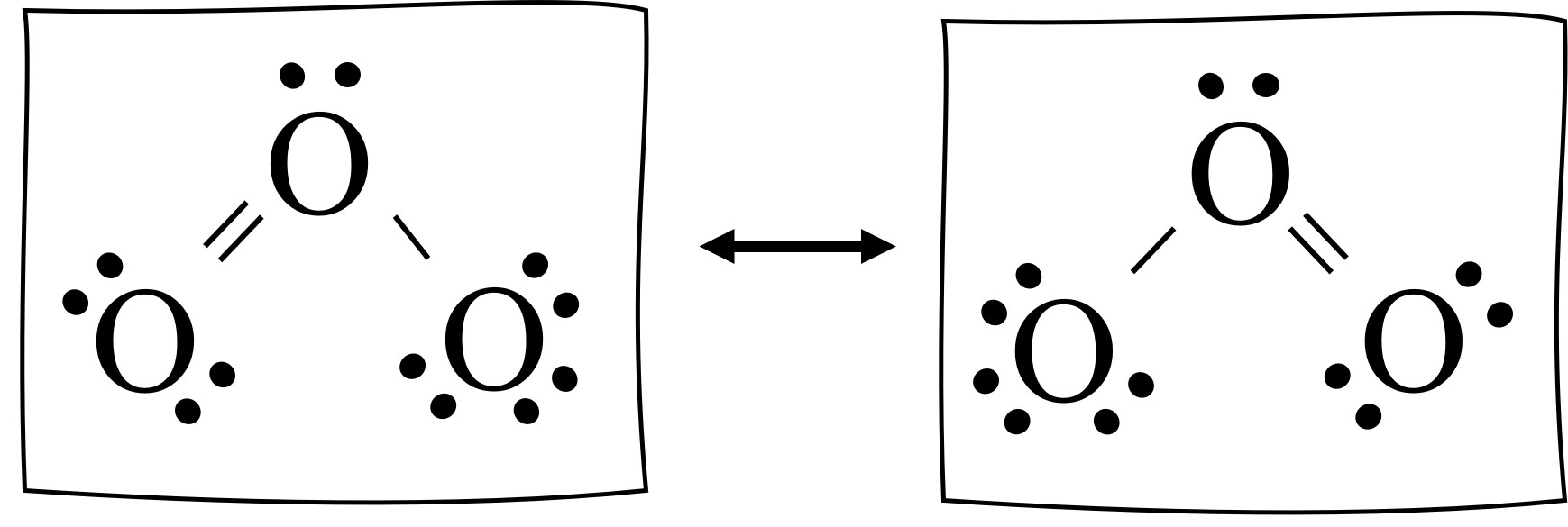
Resonance Structure
sets of Lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule.
Van der Waals Forces
Intermolecular forces (dispersion forces, dipole-dipole forces, hydrogen bonding)
Attractions between molecules
Molecular compound
composed of two or more nonmetal atoms that share electrons with one another in a covalent bond.
Unshared pair (lone pair)
a pair of electrons in an atom's outermost shell that are not shared with another atom
Formula Unit
the chemical formula for the smallest unit of a non-molecular substance, such as an ionic compound or a metal