isotopes; ions and ionic bonds
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
isotopes
atoms of the same element that have the same number of protons but different numbers of neutrons
subatomic particle question
number of protons = 16
number of neutrons = 20
number of electrons = 16+2 = 18
2- charge means it gained electrons

relative atomic mass question
(6×10)+(7×90)=690
690/100=6.9
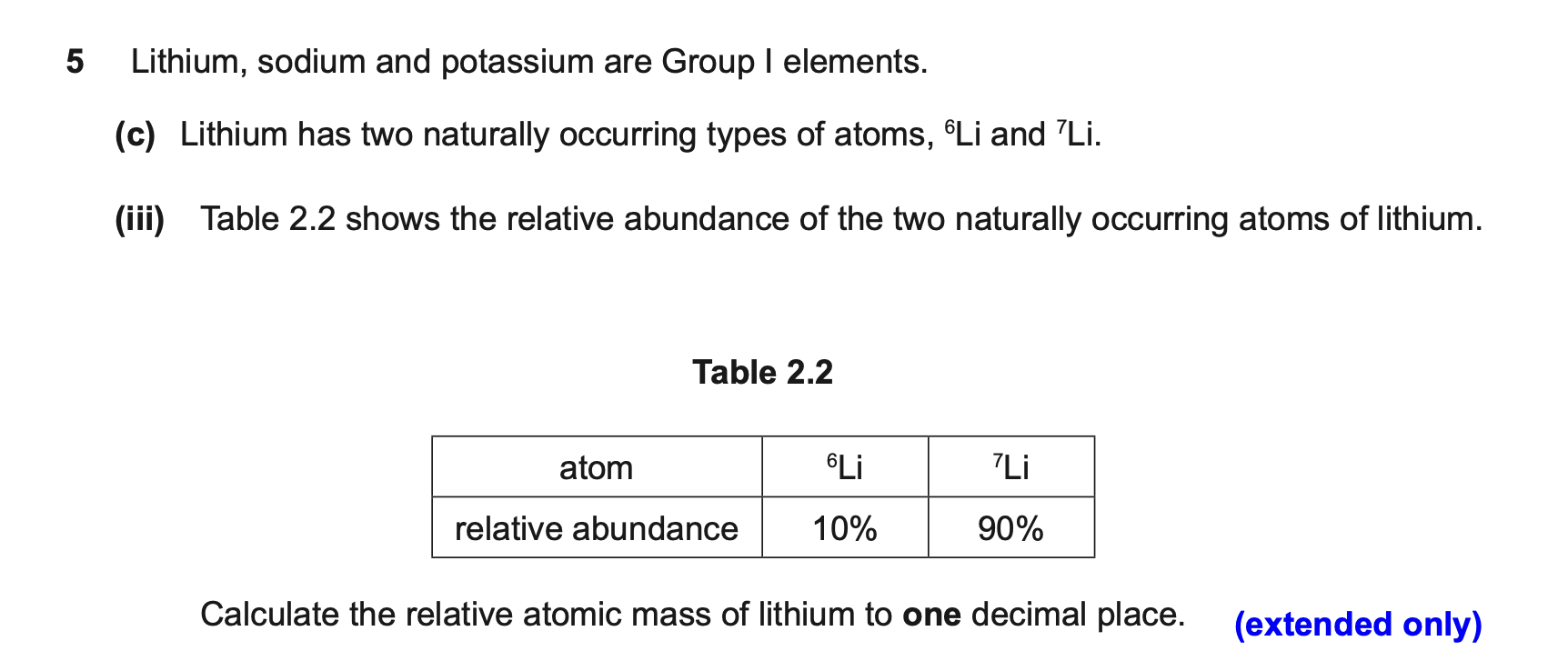
relative atomic mass formula
relative abundance always equals 100%
hydrated ion
a substance that is chemically combined with water
ionic compound properties
high melting/boililng point
cannot conduct electricity when solid (because theres no mobile ions to carry charge)
can conduct electricity in molten/aqueouss state
negative charge
missing electrons to make a full outer shell
positive charge
extra electrons preventing it from having a full outer shell
ionic bonding
a strong electrostatic attraction between oppositely charged ions
ionic bond question
why ions have a high melting/boiling point
there are strong bonds and a stable lattice structure that requires lots of energy to break apart
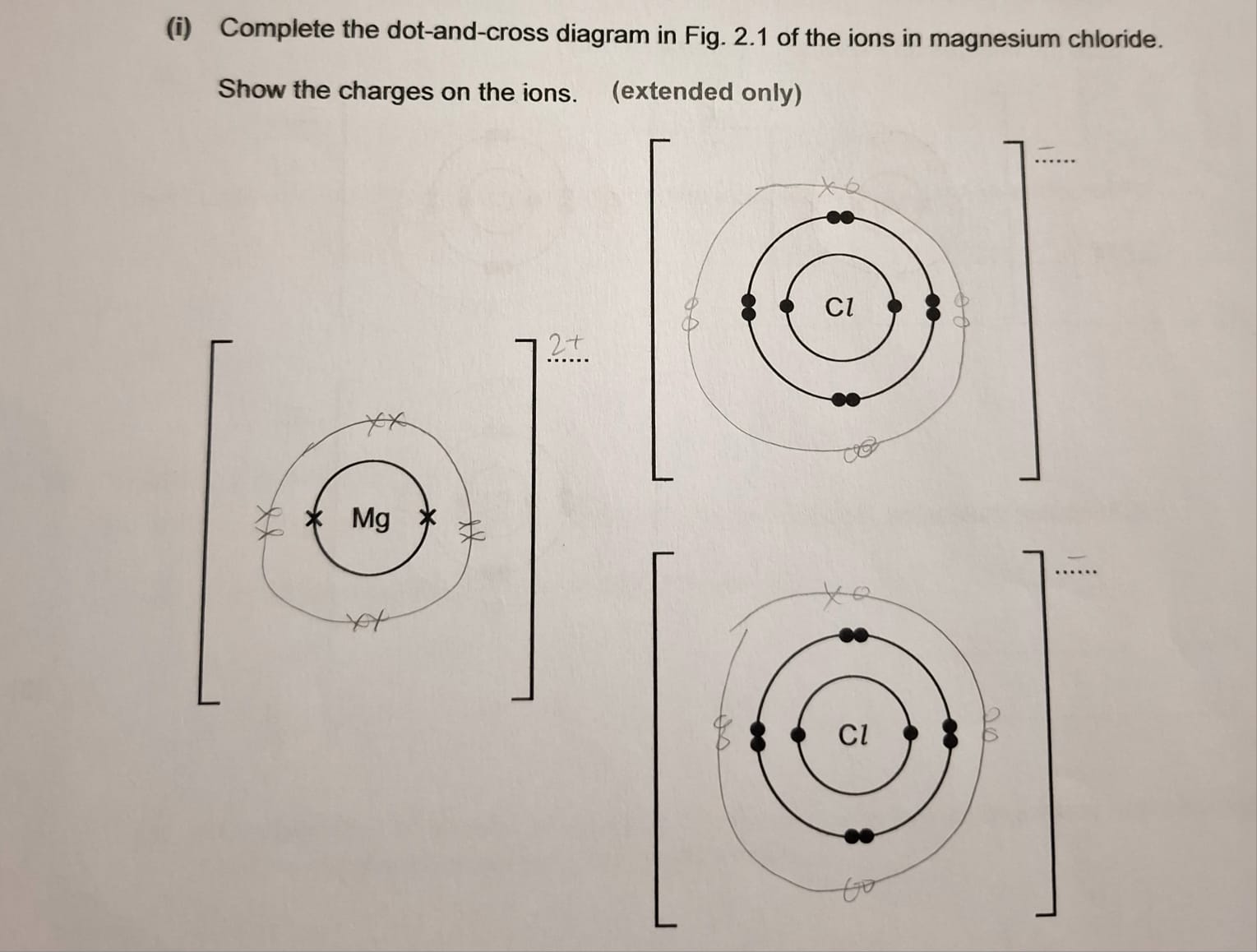
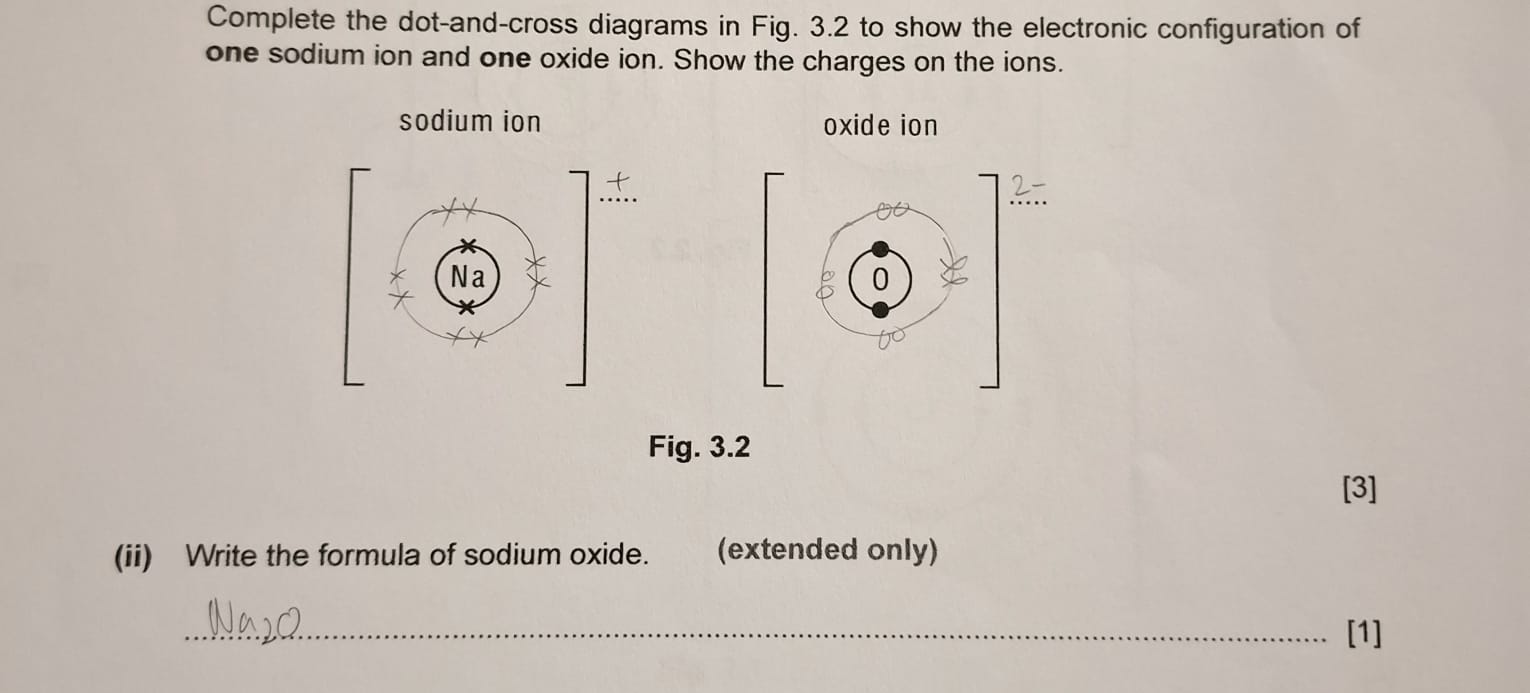
dot-and-cross diagram question