Biology - Enzymes (sem 2)
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
Metabolism
Reactions that occur inside of cells and organisms
Products of these reactions are important for cellular functioning
There two general types of metabolic reactions - ana, cata
All metabolic reactions are regulated by enzymes
Anabolic Reactions
Building reactions
When two simple form a complex
Photosynthesis is a common example
eg, CO2 + H2O → C6H12O6
Catabolic
Breaking down reactions
When a complex becomes multiple simple products
Cellular respiration is a common example
eg, C6H12O6 + O2 → CO2 + H2O
What is an enzyme?
Enzymes are specialised proteins that act as biological catalysts. They reduce the activation energyrequired for the reaction to take place, enabling them to occur at a more efficient rate.
Enzyme anatomy
enzymes have a unique shape that ensure they interact w/ specific molecules (substrates)
The key area is the active site. This is where substrates bind to allow metabolism
They are sensitive to environmental conditions. The rate of reaction changes if conditions change (tolerance ranges)
Enzymes are not consumed and are able to rebind after the reaction
Enzymes = proteins
What are the two models that explain enzyme activity?
Lock and key
Induced fit
Exergonic reactions
These reactions release energy
Referred to as downhill reactions
Always catabolic reactions
eg, When molecular bonds are broken down, energy is realeased
Endergonic reactions
Happens when molecular bonds are formed
Referred to as uphill reaction
eg, photosynthesis
Enzyme binding
For enzymes to be able to complete their reaction on a substrate they must bind with it.
The active site is where the substrate will bind and be metabolised
Enzymes are specific with which substrate they interact with and to explain this behaviour, two models exist.
Lock and Key Model
The lock and key model states that the enzymes active site has a specific shape and will only interact with substrates that fit the exact shape
This model limits the number of substrates enzymes can interact with
With enzymes being this specific it results in biochemical pathways and metabolic reactions requiring many enzymes
Lock and Key Diagram
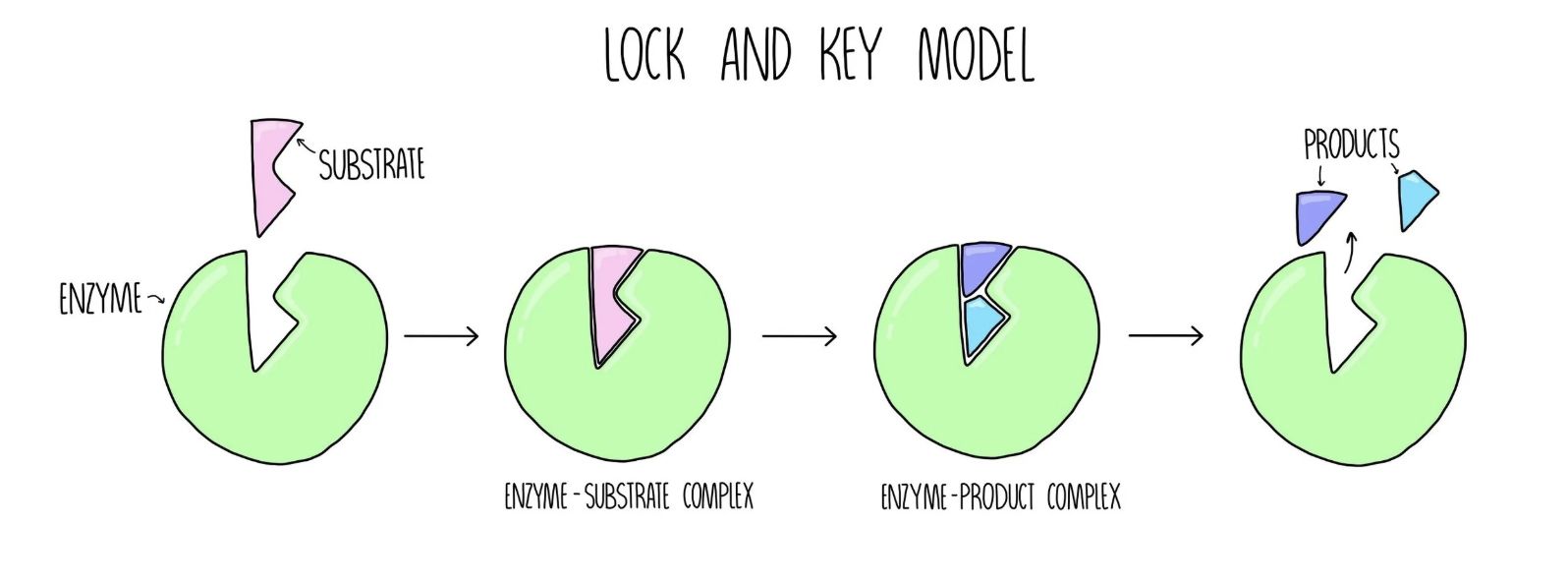
Induced Fit Model
The enzyme active site is more flexible and results in the enzyme being able to interact with many substrates
The enzyme can therefore be involved in many reactions due to the variety of substrates it can interact with.
Induced Fit Diagram
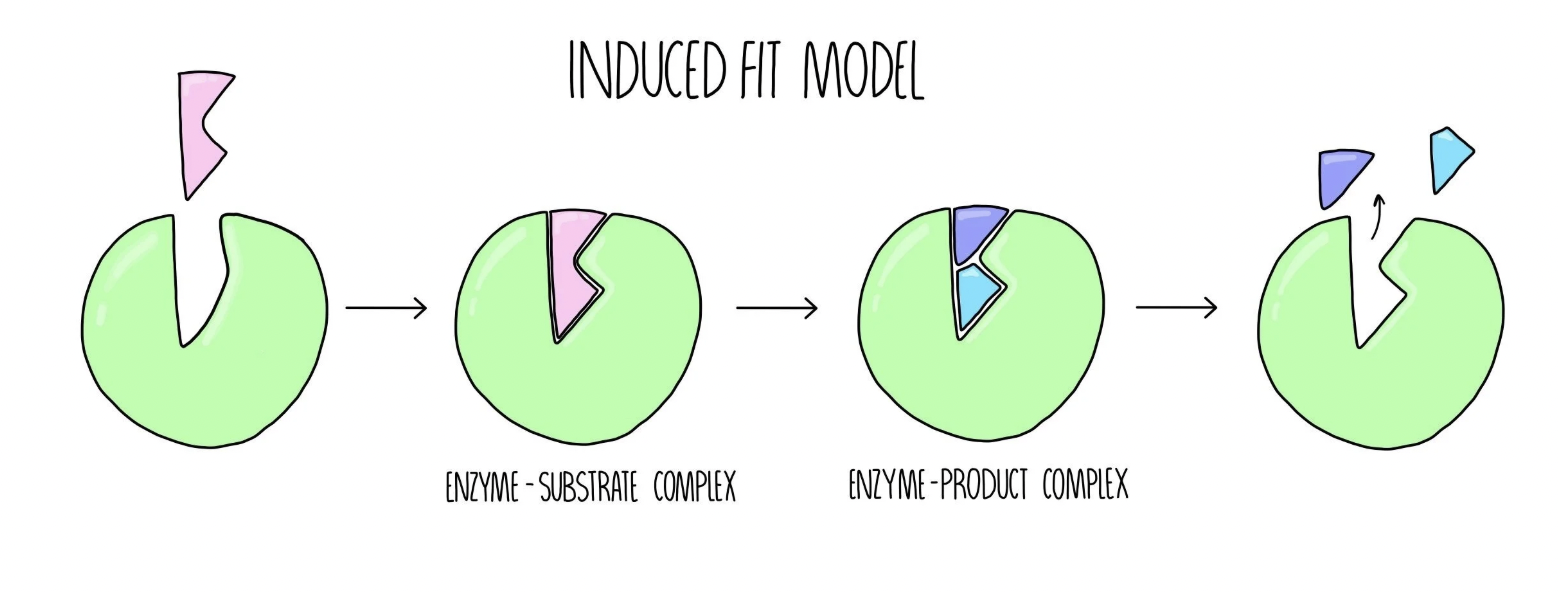
Environmental Influence on Enzyme
Enzymes are sensitive to environmental conditions. This sensitivity stems from the organisms evolution and tolerance ranges.
Sensitivity impacts the reaction rate (impacting metabolism and impacting survivability of the organism)
What are the main environmental factors?
Temp
pH
a) Substrate concentration
b) Enzyme concentration
Inhibitors
Competitive
Non competitive
Environmental influence - 1. Temperature
Enzymes have optimal temperatures where the reaction rate is at its highest
Temp can impact enzyme activity by increasing or decreasing it. As temp reduces (lowers), the kinetic energy of the objects (enzyme and substrate) decrease, reducing collisions and reducing the enzyme reaction.
So, as temp increases, the kinetic energy increase, therefore increasing reaction rate. If temp increases beyond the optimum, the enzyme is at risk of denaturing.
Denaturing
This is the irreversible change to an enzymes functional shape preventing the binding of substrates to enzymes active sites.
Temp - In Relation to the 4 Levels of Structure.
Primary structure
Not affected by theat as petife chains are strong and usually stay intact
Second structure
Broken down by heat, this is due to disrupting hydrogen bonds
Tertiary structure
Disrupted by heat
Quaternary structure
Disrupted as heat breaks non-covalent interactions holding subunits together.
Environmental influence - 2. pH
The solution surrounding an enzyme and substrate can have differing levels of acidity or alkalinity
This impact an enzymes shape as deviations impacts the bonding that occurs within an enzyme and can lead to denaturing
Most enzymes have an optimum pH at or close to neutral, however, theure are some unique cases such as pepsin in the stomach which has an optimum of 1, and amylase in saliva has an optimum of 7.
Deviations in pH can impact substrate shape and prevent binding to required enzymes, therefore lowering r.r.
Environmental influence - 3. Substrate and Enzyme concentration
Amount of substrate and enzyme limits how much product is produced in a reaction
More substrate leads to more product until all enzymes are working at full capacity
Increasing enzyme concentration causes product levels to rise exponentially until the substrate is all used up or products starts to inhibit the enzyme
The reaction rate is proportional to enzyme concentration as long as there is excess substrate
Enzyme concentrations are regulated by cells based on needs. This happens through
controlling enzyme production
breaking down enzymes
activating enzymes in response to stimuli
Cofactors and Coenzymes
Some enzymes are inactive until they bind with other molecules or ions that change their shape
This changes the active sites shape and charge, allowing it to better bind substrates and catalyse reactions
Two types of substances can help activate enzymes
Cofactors
small inorganic substances
Coenzymes
small, non protein molecules
required for enzyme activity
act as carriers to and from reactions
often made from dietary vitamins
Environmental influence - 4. Inhibitors
Inhibitors are molecules that reduce reaction rate by preventing enzymes from interacting with substrates
Two types exist
Competitive
Non Competitive
Competitive Inhibitor
The competitive inhibitor will bind to the enzymes active site, it then blocks the substrate from binding and reduces the reaction by preventing the formation of the enzyme - substrate complex.
Competitive Inhibitor - Diagram
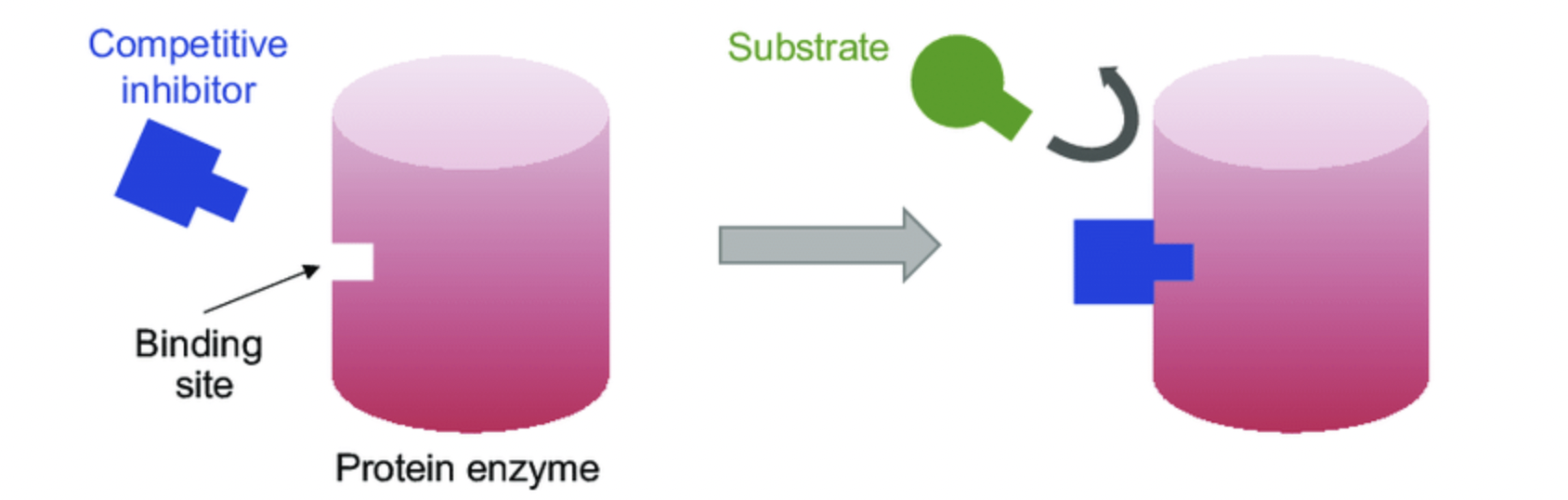
Non Competitive Inhibitor
Is a molecule that binds with the enzyme, away from the active site, but sue to the formation of new bonds, the enzyme active site changes shape
As the active site shape changes the substrate will no longer be able to bind, therefore an overall reaction rate
Non Competitive Inhibitor - Diagram
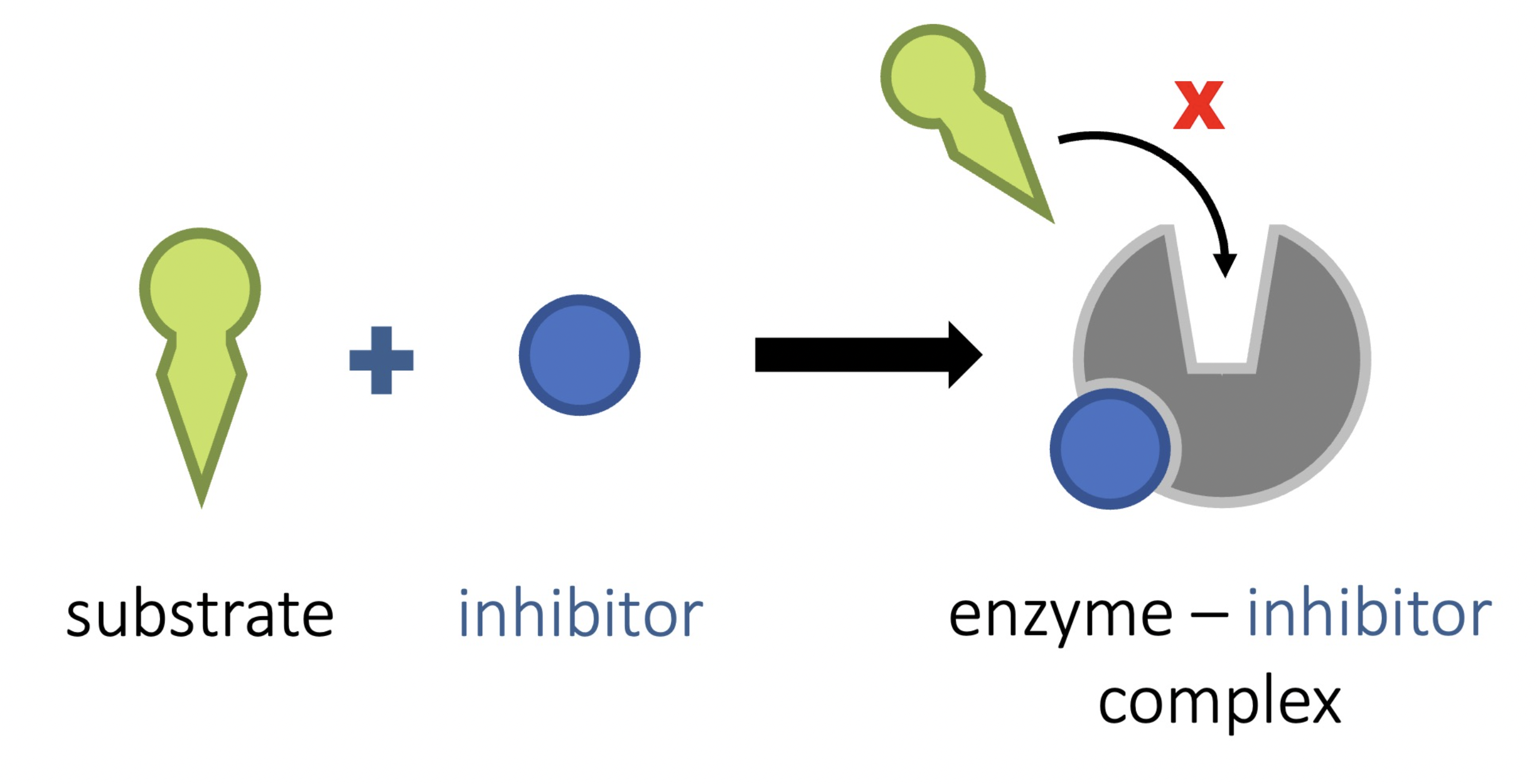
Common Inhibiotors
Penicillin
Aspirin
Mercury
Penicillin - Competitive
Target enzyme - Transpeptidase
Mechanism:
Penicillin mimics the substrate and binds the the active site
Forms a covalent bond, permanently deactivating it
Effect:
Weakens bacterial cell walls leading to cell lysis
Aspirin - Competitive
Target enzyme - Cyclooxygenase
Mechanism:
Blocks prostaglandin synthesis (causing pain)
Effect:
Reduces pain, fever, and inflammation
Mercury - Non competitive
Target enzyme - Any enzyme with sulfhydryl
Mechanism:
Mercury binds tightly to sulfhydryl groups, causing enzymes to denature or lose function
Effect:
Disrupts cellular metabolism, especially in nervous tissue
Causes symptoms of mercury poison
Why would enzyme action be inhibited?
Medications inhibit enzymes to block unwanted processes
Common Enzymes
Cellulase
Amylase
Protease
Lipase
Cellulase
Some cellulase can be found in small amounts in the gut, from gut micro bacteria
The role of cellulase is to break down cellulose into simple sugars like glucose or cellobiose
In humans most cellulose passes through undigested acting as dietary fibre
Amylase
Amylase is an enzyme produced in the salivary glands to produce salivary amylase (ptyalin). It is also produced by the pancreas to produce pancreatic amylase
Amylase breaks down starch (polysaccharide) into simpler sugars like maltose (disaccharide), and eventually glucose (monosaccharide).
Protease
Protease is produced by and formed in the stomach → pepsin, the pancreas → trypsin + chymotrypsin, and small intestine lining.
Protease breaks down proteins into smaller peptides and amino acids, which the body can absorb and use to build own proteins
Lipase
Lipase is produced by and found in the pancreas → pancreatic lipase, the mouth → small amounts of lingual lipase, and the stomach to form → gastric lipase.
The role of lipase is to break down lipids (fats and oils) into glycerol and fatty acids