Neural Development
1/77
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
78 Terms
Initiation of neural development is dependent on…
gastrulation
Gastrulation
local invagination of a subset of cells in the very early embryo
once complete the embryo consists of 3 layers of cells called germ layers
3 germ Layers
Ectoderm
Mesoderm
Endoderm
Ectoderm
Outer layer
Mesoderm
Middle layer
Endoderm
Inner layer
What is neurulation and what does it produce?
Is the process where the neural plate forms and folds into the neural tube, giving rise to the brain, spinal cord, and most of the PNS.
What structure initiates neural development and defines embryonic midline symmetry?
The notochord
What are neural crest cells and what do they give rise to?
Cells from the lateral edges of the neural plate that migrate and form parts of the PNS and non-neuronal structures like pigment cells and facial cartilage.
Gastrulation defines
Midline
Anterior-Posterior axes
Dorsal-Ventral axes
Notochord forms where?
at the midline of the gastrulating embryo
this is a central event for the development of the nervous system
Generated by the primitive streak
Defines the embryonic midline and the axis of symmetry for the entire body
Neuroectoderm
lies immediately above the notochord; gives rise to the entire nervous system
Notochord sends inductive signals to the overlying ectoderm to cause a subset of cells to differentiate into…
neuroectodermal precursor cells (neural stem cells)
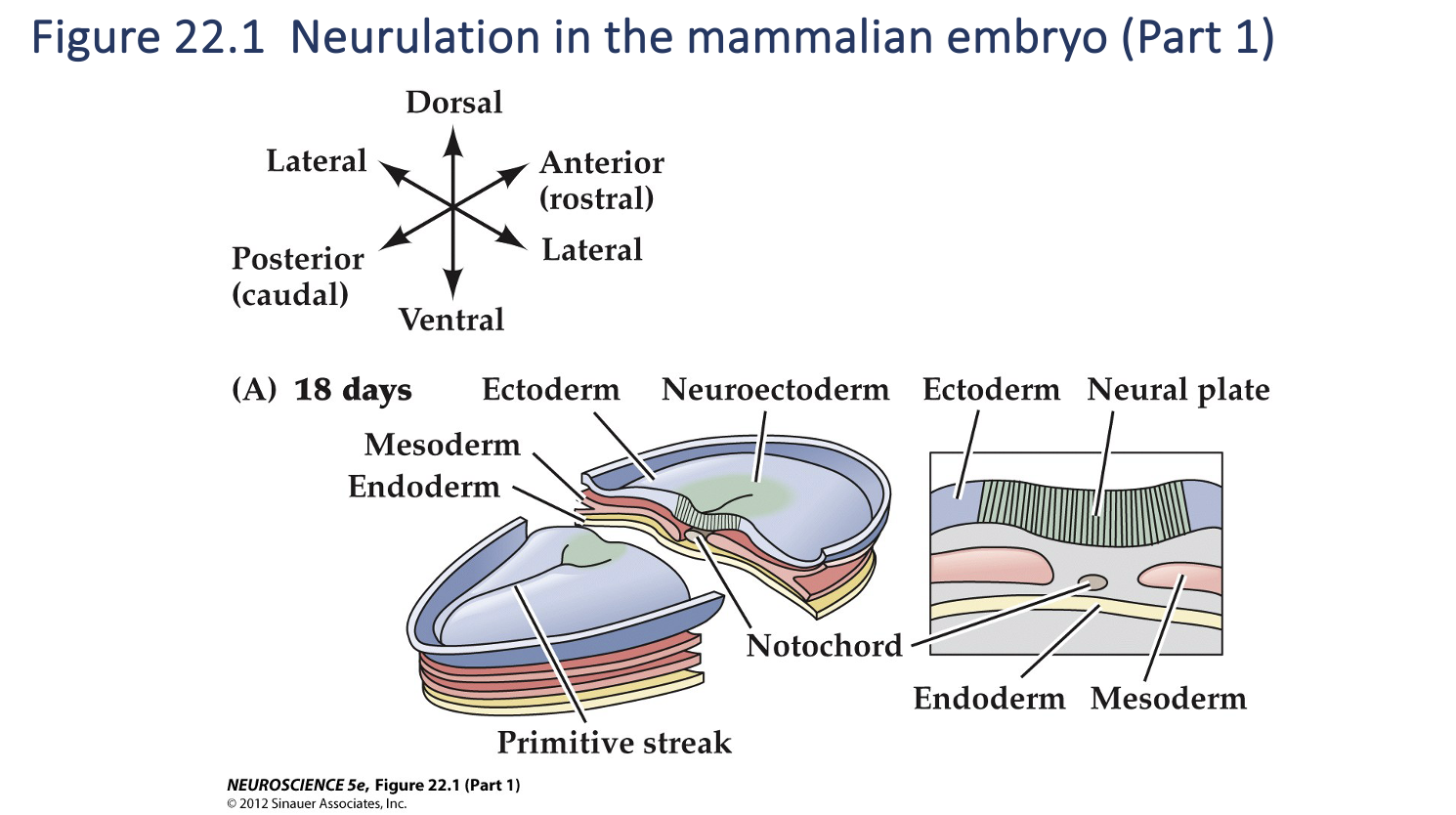
Figure 22.1- Formation of neural crest and neural tube
As neurulation proceeds, the neural plate begins to fold at the midline (adjacent to the notochord), forming the neural groove and, ultimately, the neural tube.
The neural plate immediately above the notochord differentiates into the floorplate, whereas the neural crest emerges at the lateral margins of the neural plate (farthest from the notochord). (C) Once the edges of the neural plate meet in the midline, the neural tube is complete.
Neurulation components
Neural Plate
Neural Tube
Neural Plate forms…
as a result of thickening of the neural ectoderm
Neural tube forms…
As a result of an infolding of the neural plate
gives rise to the brain, spinal cord and most of the PNS
Neuroectodermal precursor cells =
neural stem cells
Neural stem cells divide to produce more…
precursor cells
Have to capacity to differentiate into any type of cell found in nervous tissue, i.e. astrocytes, microglia, neurons etc.
Some cells of the neural tube differentiate into specialized cells that form the…
floorplate
Molecular signals from the floorplate and the notochord specify the position and fate of the spinal cord and hindbrain
Precursor cells further away from the ventral midline give rise to neurons in more
dorsal regions of the spinal cord and hindbrain; facilitated by the roofplate
The notochord, floorplate and roofplate are transient structures that…
provide signals to the developing neural tube but disappear once initial
nervous system development is complete
Neural Crest cells arise from where?
the lateral edges of the neural plate as the neural tube forms
migrate away from the neural tube through an extracellular matrix of mesenchymal cells
Neural Crest Cells give arise to…
a variety of progeny (not CNS; portions of the PNS)
• Neurons and glia of the sensory and autonomic ganglia
• Neurosecretory cells of the adrenal gland
• Neurons of the enteric nervous system
• Non-neuronal structures – pigment cells beneath the epidermis, cartilage and bone
(particularly of the face and skull)
4 populations of Neural Crest Cells
Cranial
Vaginal
Trunk
Sacral
Cranial Neural Crest Cells
Cranial sensory ganglion, non-neural cells that become the cranial skeleton, thyroid gland, and teeth.
Vagal Neural Crest Cells
Enteric nervous system
Trunk Neural Crest Cells
Dorsal Root Ganglion
Sacral Neural Crest Cells
Additional neurons of the enteric n.s. and posterior ganglion of the sympathetic chain
Neural stem cell (Ex of somatic stem cell)
Self renew
Multipotent- Upon terminal division and differentiation can give rise to all types of neural cells (neurons, astrocytes, and oligodendrocytes)
Neural stem cell can give arise to
- Other stem cells
- Or any differential cells from CNS or PNS
Neural Progenitor Cell
Can not continuously self renew
Only has the ability to give rise to only one differentiated progeny class
- Ex: An oligodendrocyte progenitor cell can only produce oligodendrocytes
Embryonic Stem Cell (ES Cells)
Can do infinite self-renewal like somatic stem cells
Pluripotent- Can give rise to all tissues and cell types in an organism
Induced Pluripotent Stem Cells (IPSCs)
They are somatic cells that have been genetically reprogrammed to behave like ES cells—i.e., to become any cell type.
They acquire this pluripotency when certain signals (genes or factors) are added during culturing
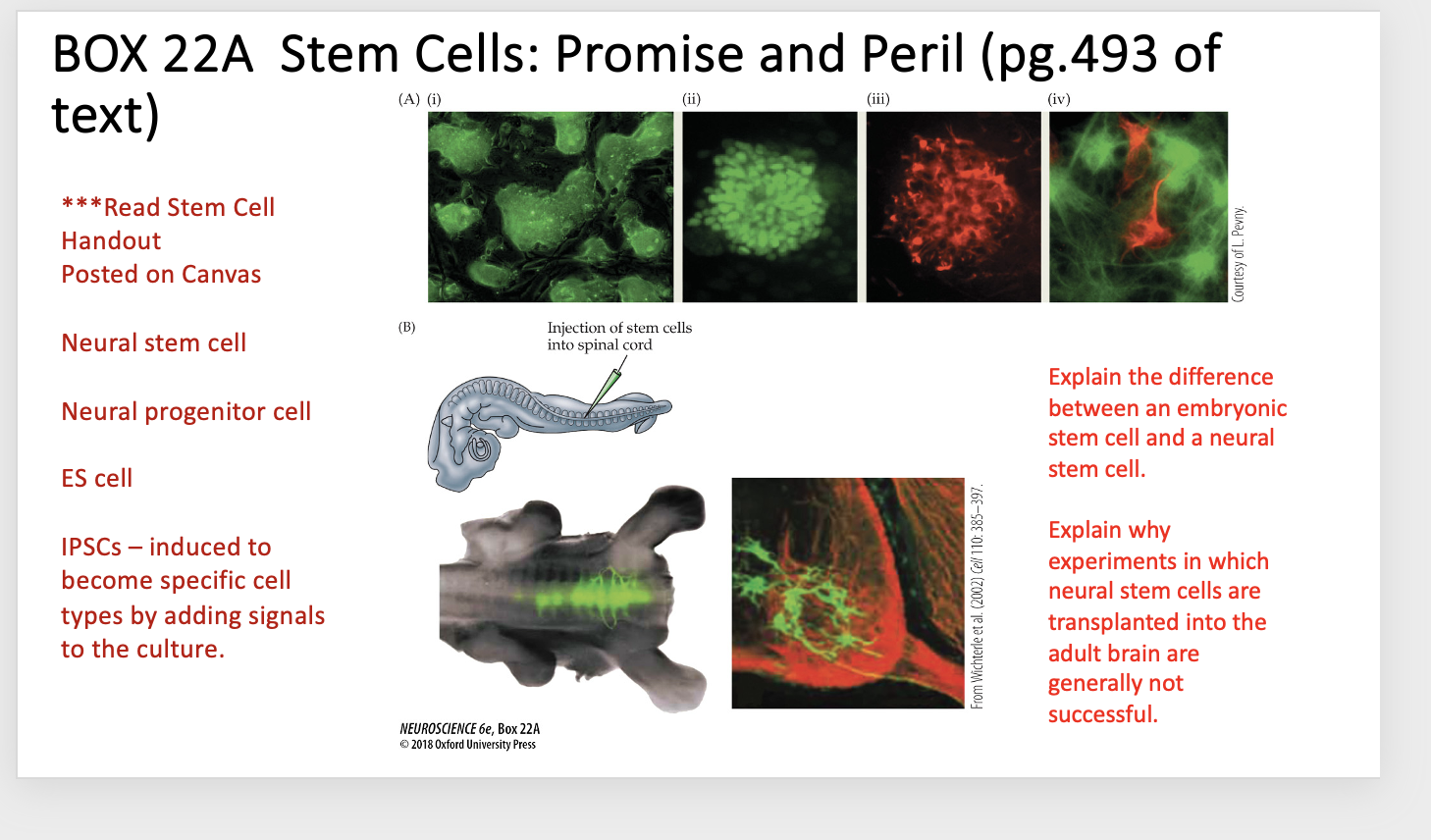
Explain Box 22A
BOX 22A Stem Cells: Promise and Peril
(A) Embryonic stem cells differentiate into various neuronal cell types.
(i) Colonies of ES cells prior to differentiation.
(ii, iii) After exposure to neuralizing signals, individual stem cell colonies express markers associated with different neural precursor cells. Cells in this colony express both green, a marker of early neural precursors, and nestin red, a marker for later neural progenitor cells.
(iv) After several days in culture, both neurons (red, labeled for neuron-specific tubulin) and astrocytes (green, labeled for glial fibrillary protein) have been generated from ES cells.
(B) Left top: Injection of fluorescently labeled embryonic stem cells into the spinal cord of a host chicken embryo shows that ES cells integrate into the host spinal cord and apparently extend axons.
Below: the progeny of the grafted ES cells are seen in the ventral horn of the spinal cord. They have motor neuron-like morphologies, and their axons extend into the ventral root.
Explain the difference between an embryonic stem cell and a neural stem cell
Embryonic stem cells (ES cells) are pluripotent: they can become any cell type in the body and can self-renew indefinitely.
Neural stem cells are more limited: they can only give rise to cell types within the nervous system (neurons and glia), though they also can self-renew
Explain why experiments in which neural stem cells are transplanted into the adult brain are generally not successful
The adult brain doesn’t provide the right developmental signals for the stem cells to differentiate properly.
The transplanted cells need specific molecular cues (e.g., from embryonic development) to become the correct type of neuron or glial cell, and the adult brain environment usually doesn’t provide those signals.
As a result, they may fail to integrate, differentiate, or survive effectively.
Somite
Segments of mesoderm that lie alongside the neural tube and give rise to skeletal muscle, vertebrae and dermis
Mesenchymal cells
Segments of mesoderm that lie alongside the neural tube and give rise to skeletal muscle, vertebrae and dermis
Olfactory placode
A platelike thickening of embryonic ectoderm from which a definitive structure develops
Formation of major brain subdivisions: Following the formation of the neural tube, beginnings of the major brain regions start to appear when?
bending, folding and constriction of the neural tube
3 Initial Subdivisons of the neural tube- Primitive regions
Prosencephalon
Mesencephalon
Rhombencephalon
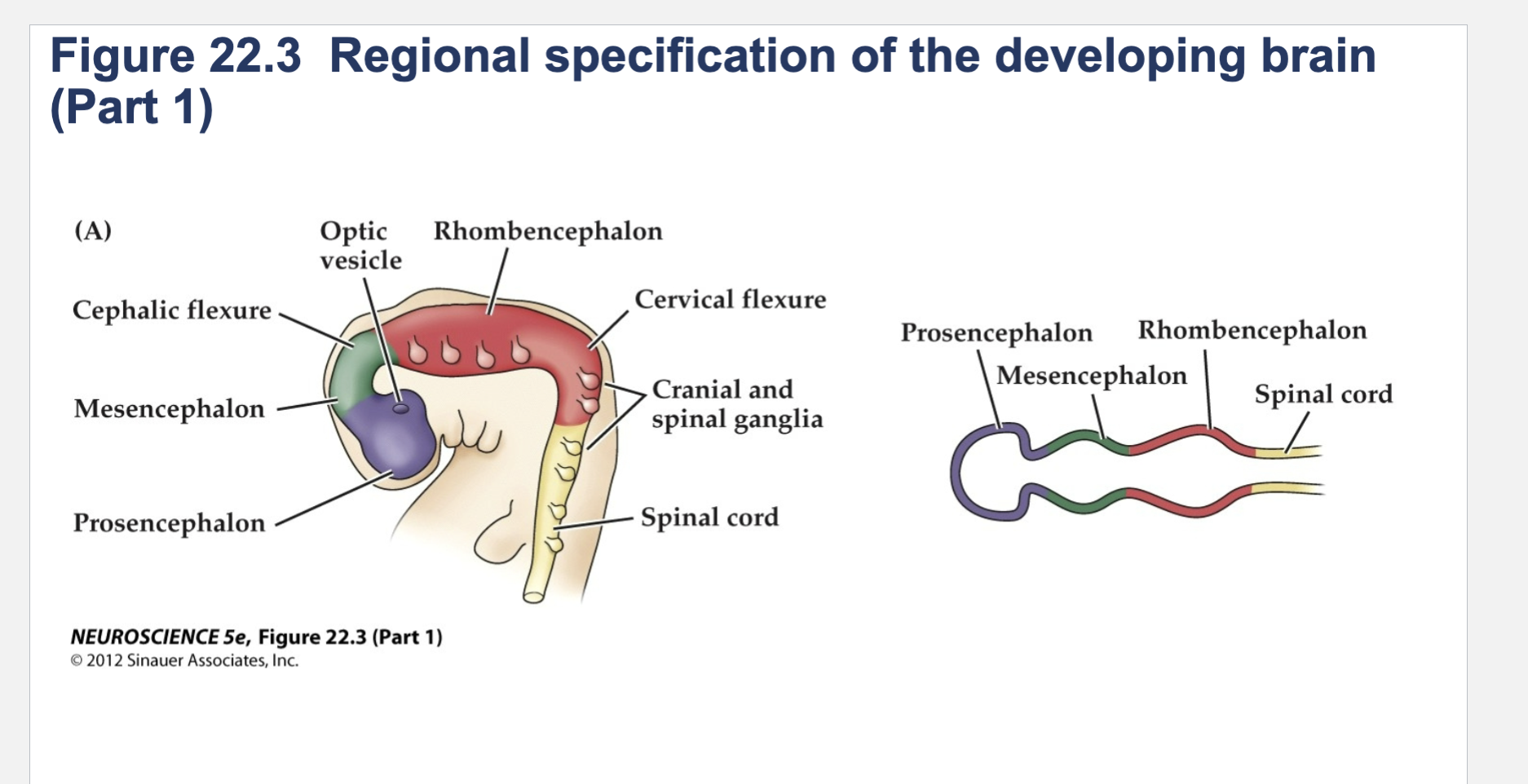
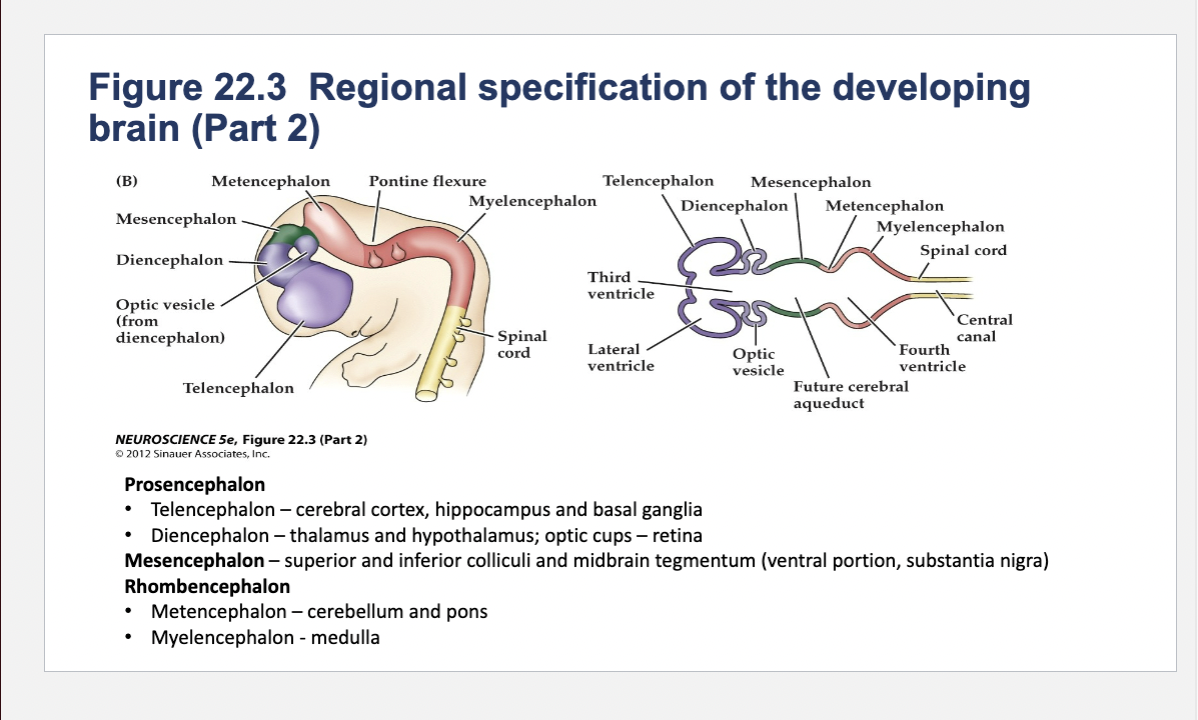
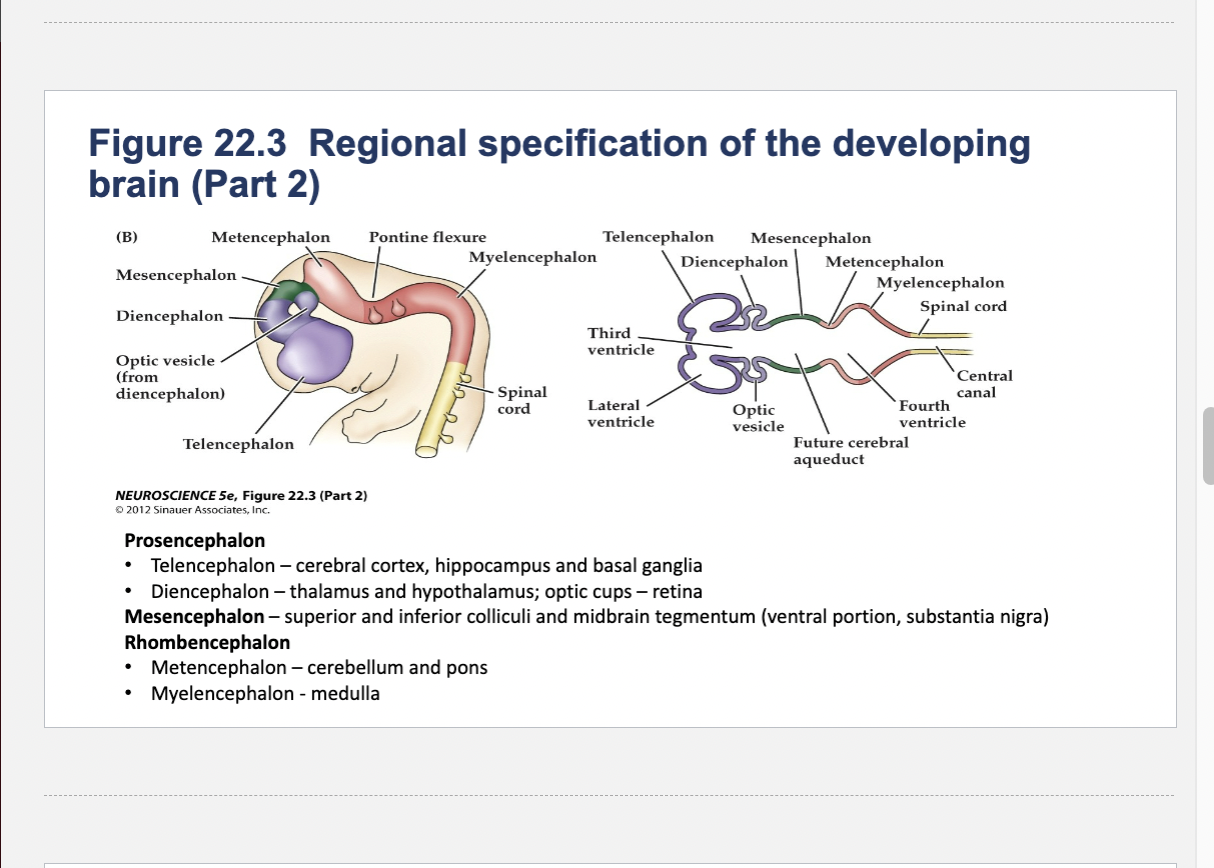
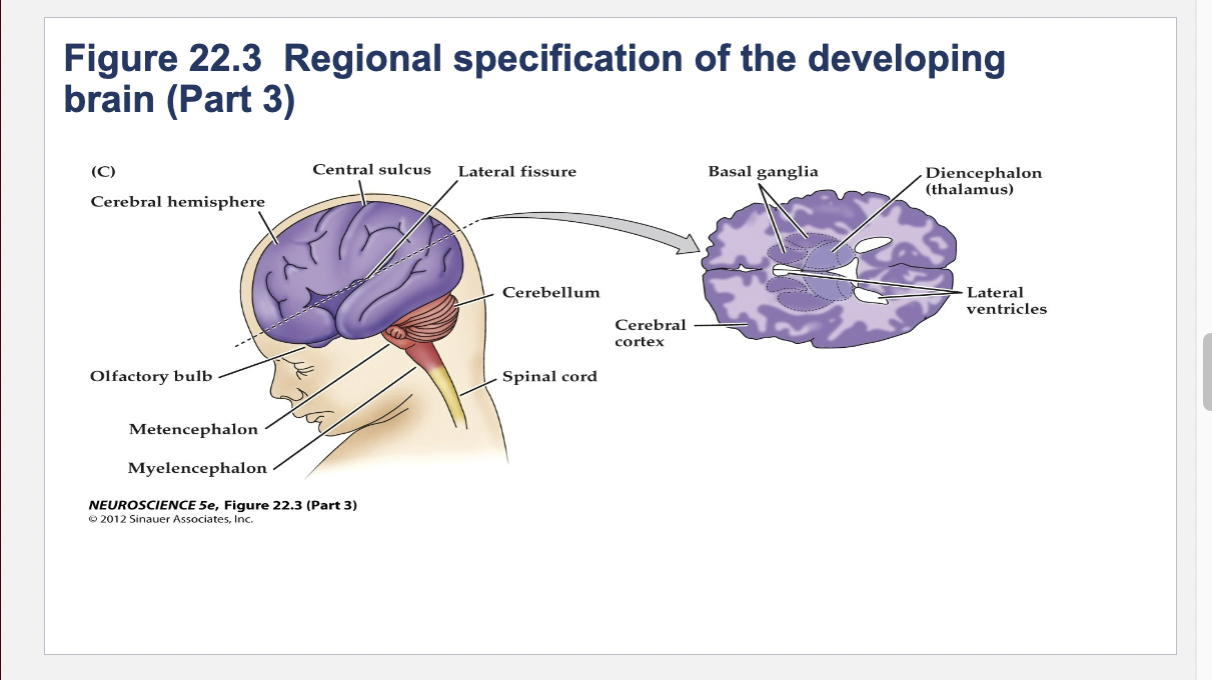
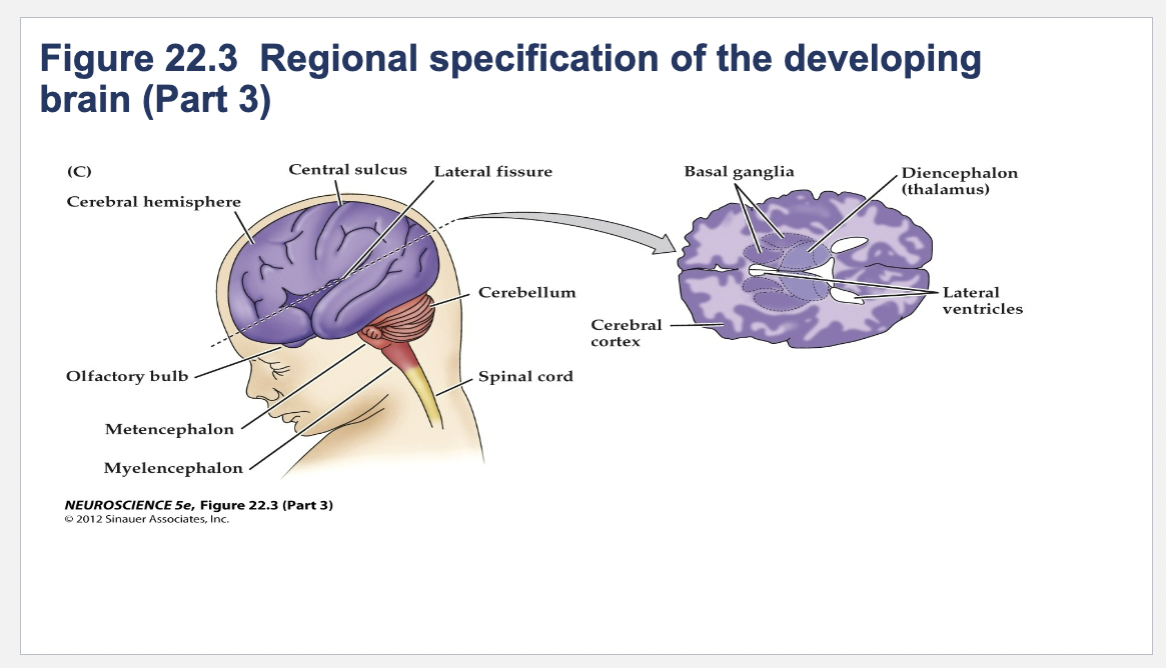
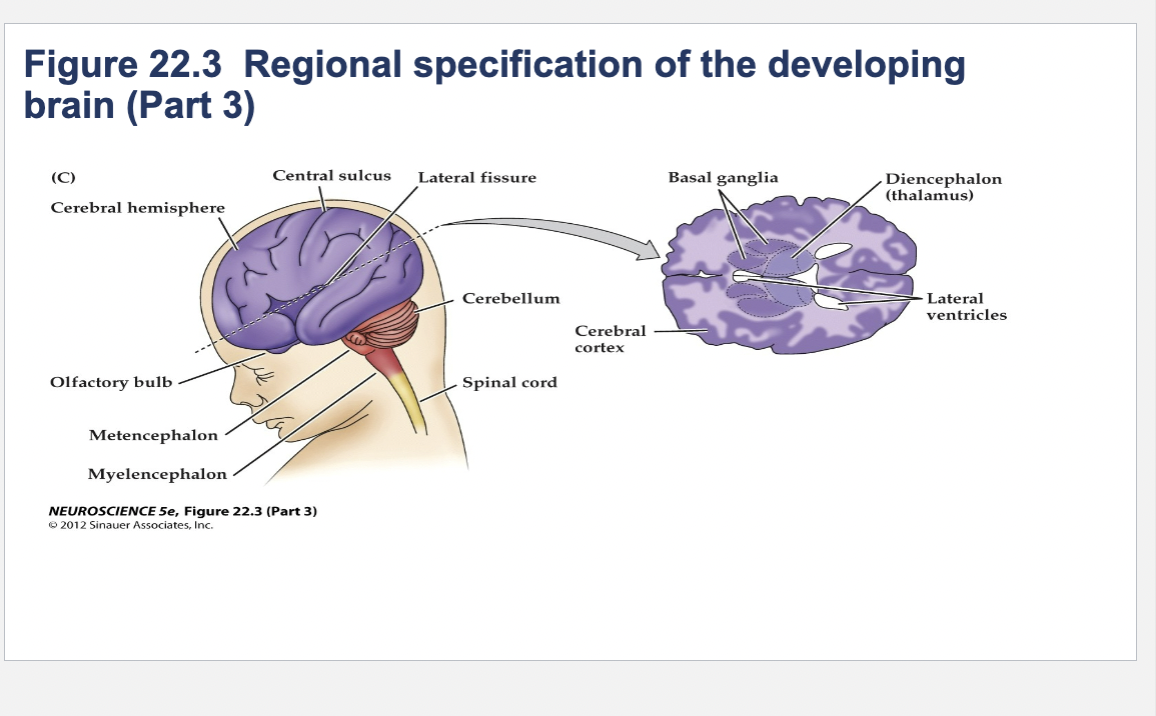
Figure 22.3- Regional specification of the developing brain.
(A) Early in gestation the neural tube becomes subdivided into the prosencephalon (at the anterior end of the embryo), mesencephalon, and rhombencephalon. The spinal cord differentiates from the more posterior region of the neural tube. The initial bending of the neural tube at its anterior end leads to a cane shape. At right is a longitudinal section of the neural tube at this stage, showing the position of the major brain regions. (B) Further development distinguishes the telencephalon and diencephalon from the prosencephalon; two other subdivisions—the metencephalon and myelencephalon—derive from the rhombencephalon. These subregions give rise to the rudiments of the major functional subdivisions of the brain, while the spaces they enclose eventually form the ventricles of the mature brain.
At right is a longitudinal section of the embryo at the developmental stage shown in (B).
(C) The fetal brain and spinal cord are clearly differentiated by the end of the second trimester. Several major subdivisions, including the cerebral cortex and cerebellum, are clearly seen from the lateral surfaces. At right is a cross section through the forebrain at the level indicated showing the nascent sulci and gyri of the cerebral cortex, as well as the differentiation of the basal ganglia and thalamic nuclei.
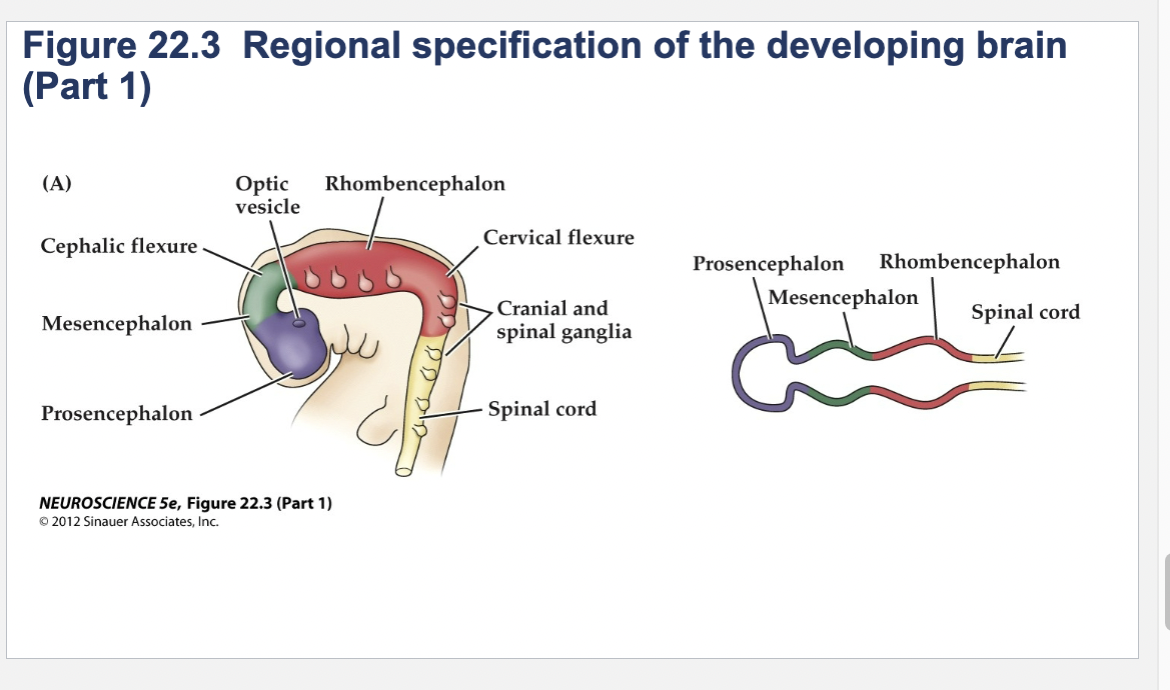
Prosencephalon
forebrain
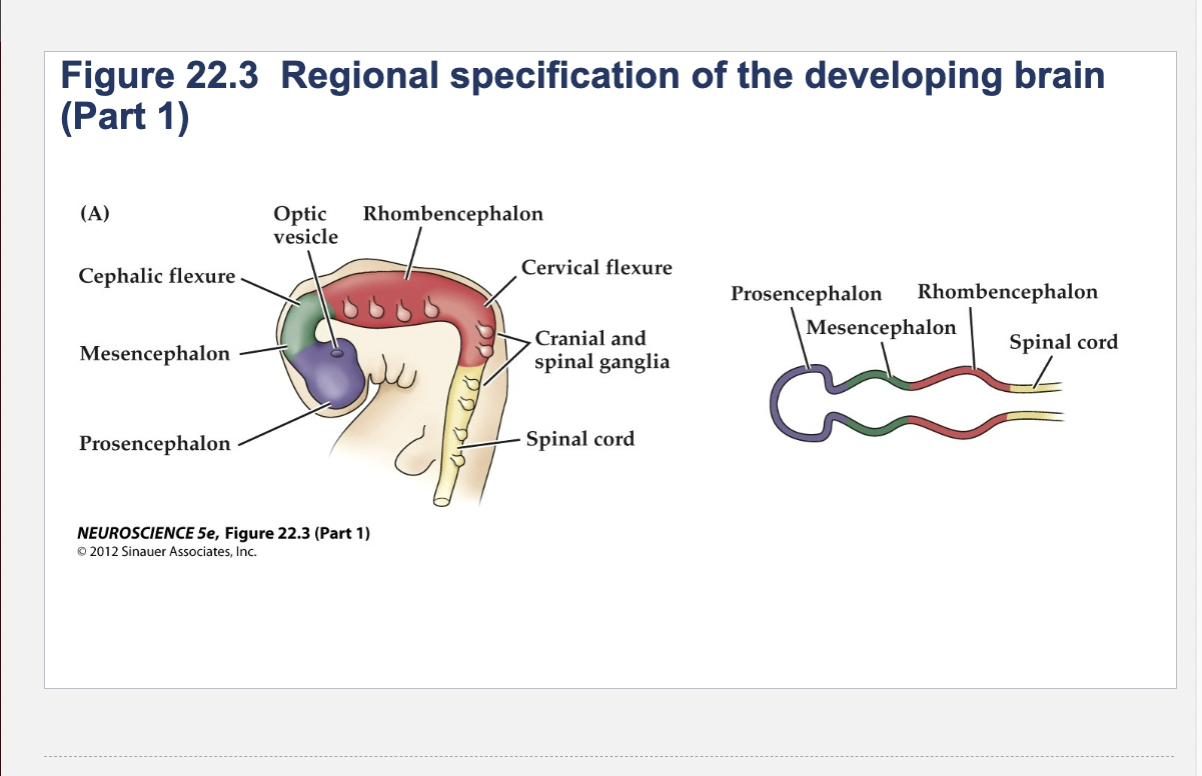
Mesencephalon
midbrain
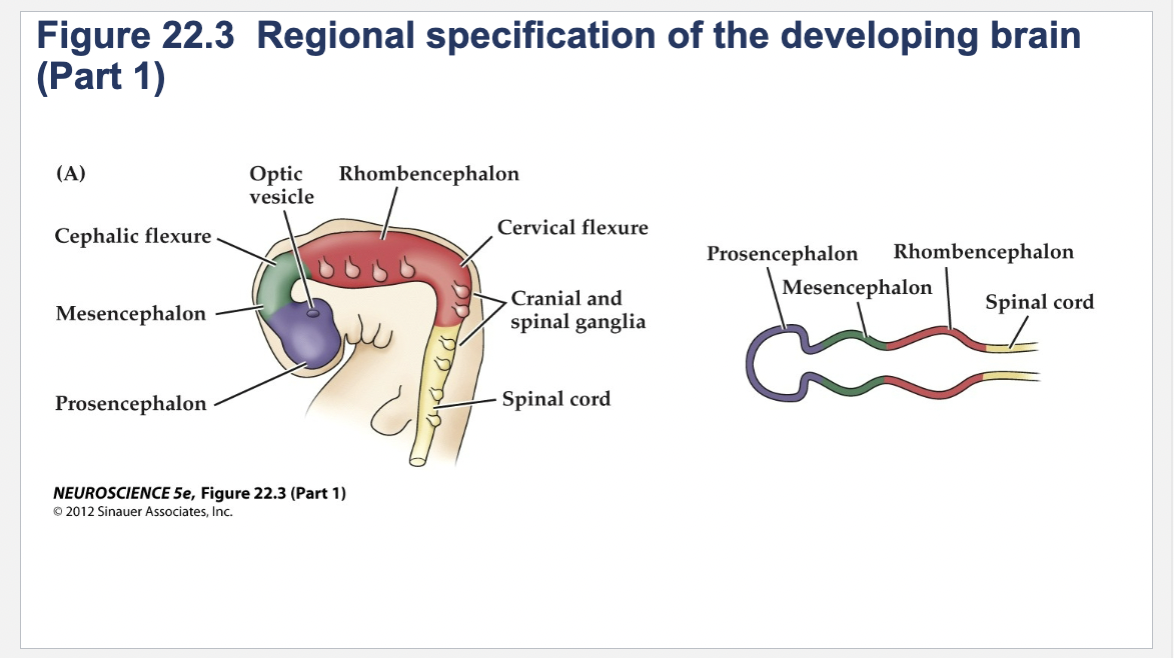
Rhombencephalon
hindbrain
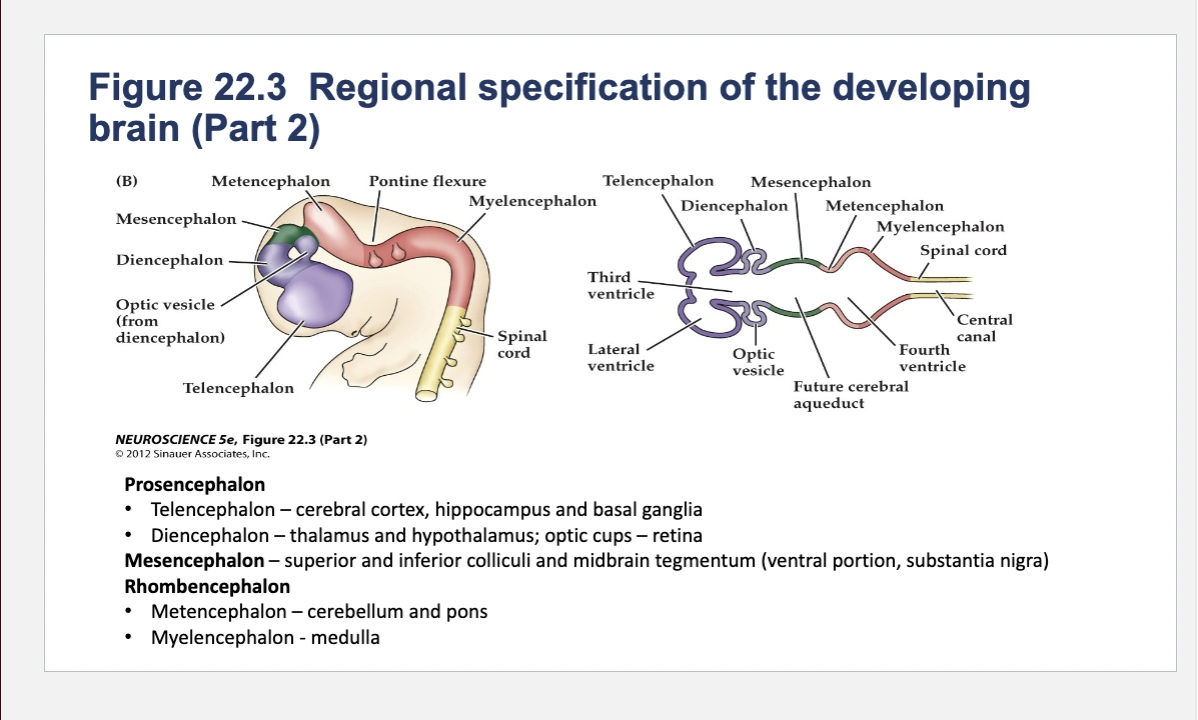
Components of Prosencephalon (Forebrain)
Telencephalon
Diencephalon
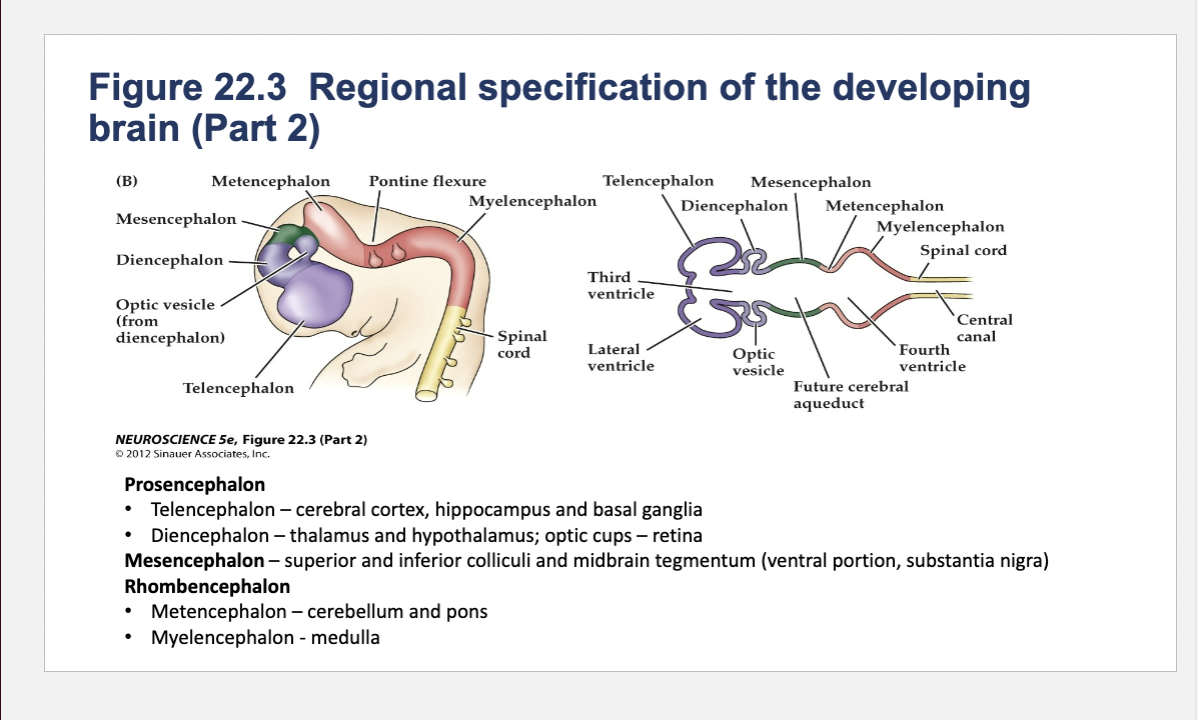
Telencephalon components
cerebral cortex, hippocampus and basal ganglia
Diencephalon components
thalamus and hypothalamus; optic cups – retina
Components of Mesencephalon (midbrain)
superior and inferior colliculi and midbrain tegmentum (ventral portion, substantia nigra)
Components of Rhombencephalon (hindbrain)
Metencephalon
Myelencephalon
Metencephalon components
cerebellum and pons
Myelencephalon
medulla
How do neural stem cells know what cell type to differentiate into?
Early experiments – precursor cells moved to a different regions, results differ
depending on timing and location
• Acquire the identity of the new region (receive instructions based on new location)
• Retain an identity that reflects their original position
• Cells that are removed are compensated by local cell proliferation – little disruption to development
• Absence of cells disrupts subsequent development
• Relocation of cells causes a complete change in the local developmental program
• Suggested that neural induction relies on signals provided by adjacent cells or tissues – not proven until the 1990’s
• Molecular signals are secreted by one embryonic cell class and tissue and then diffuse and act on an adjacent cell class or tissue (ex. Roofplate, floorplate)
• Graded effects – based on distance from source of signal
• More specific effects – at boundaries between distinct cell populations
Cell Proliferation
Cell grows and divides
Important because you need to have enough cells to create structures
Cell Migration
Movement of cell depending on the chemical signal
Signal come from extracellular environments
Cell Differentiation
Unspecialized cells becoming specialized
Gene Expression- Genes that are transcribed to make proteins
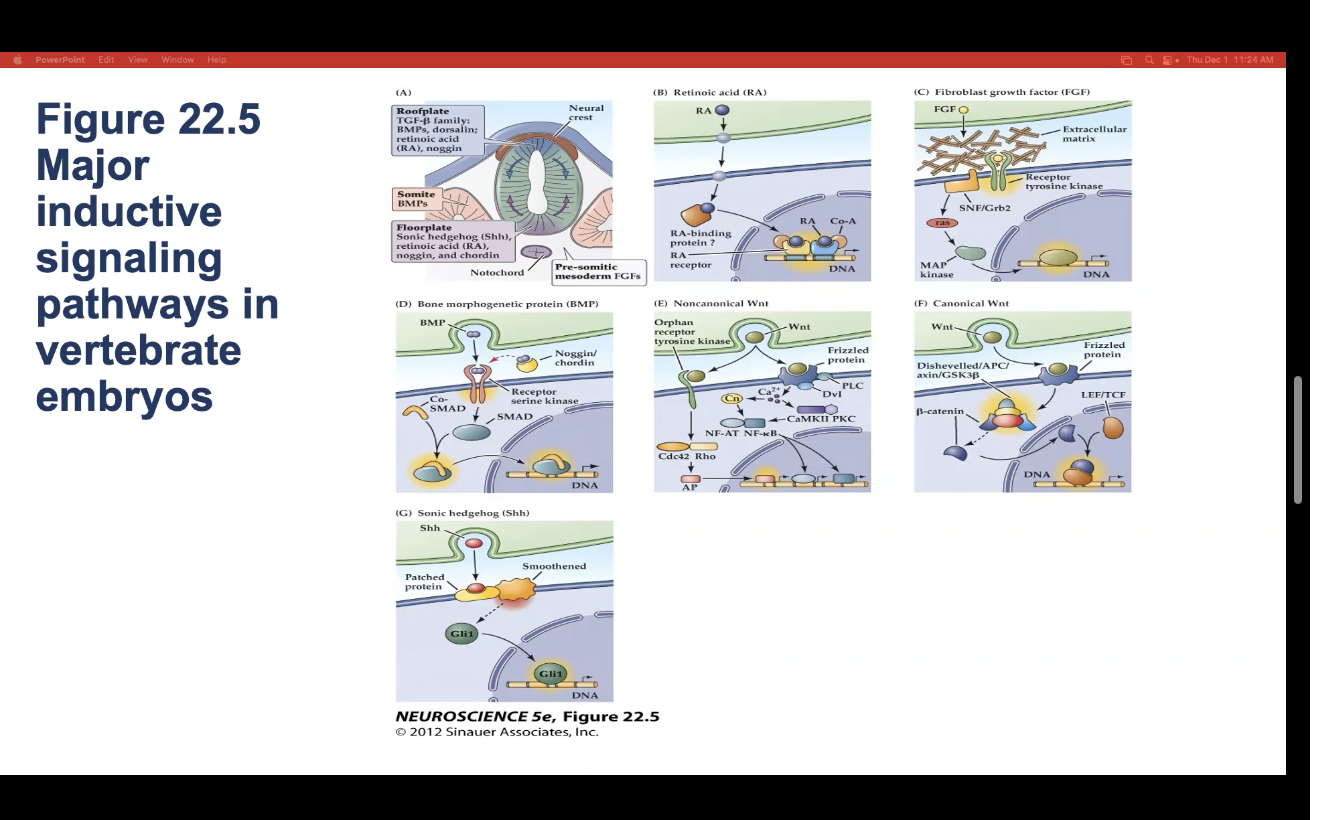
7 types of major inductive signaling pathways in vertebrate embryos
Roofplate
Retinoic Acid
Fibroblast Growth Factor (FGF)
Bone Morphogenetic Protein (BMP)
Noncanomical Wnt
Canomical Wnt
Sonic Hedgehod (Shh)
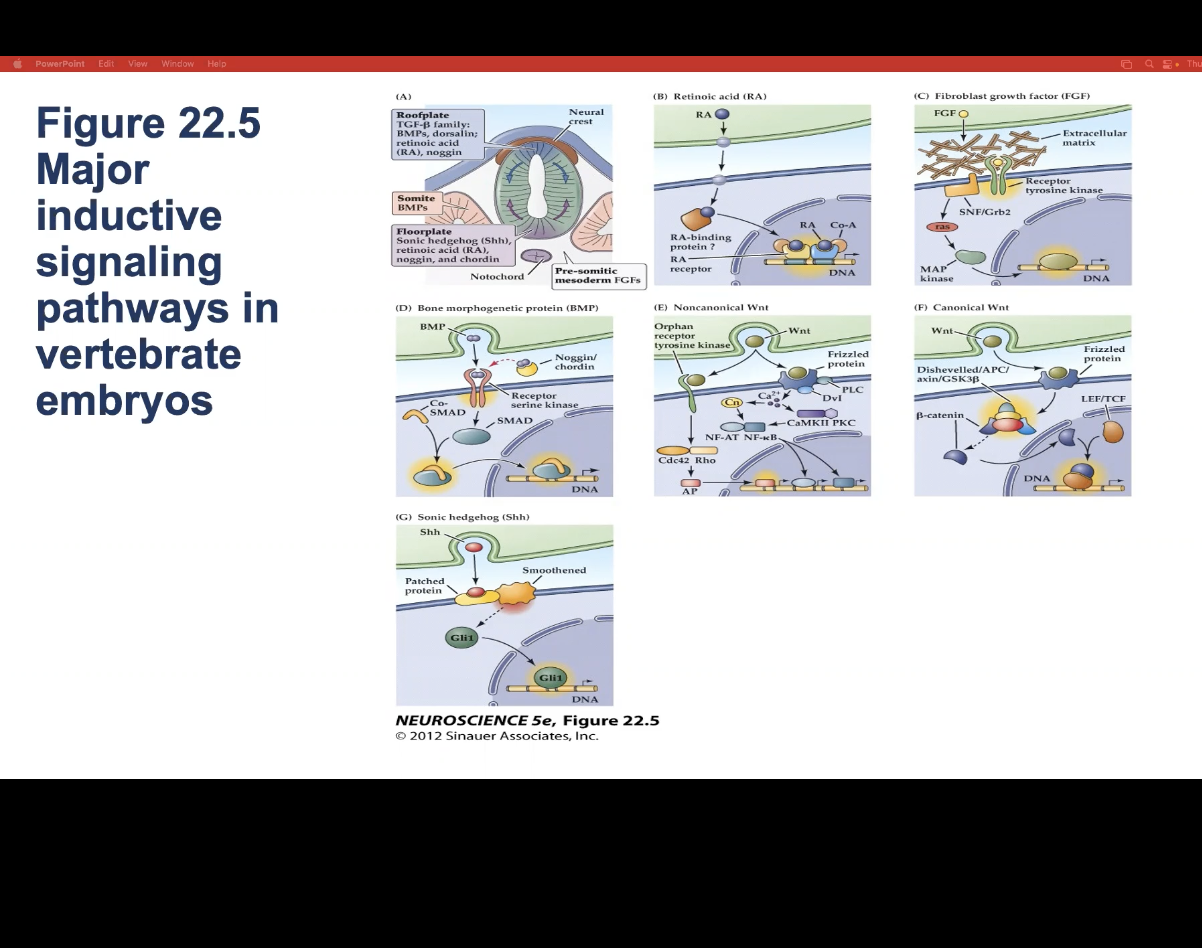
How do do all major inductive signaling pathways induce neuronal precursor cells to differentiate?
By altering transcription of specific genes and thus changing gene expression
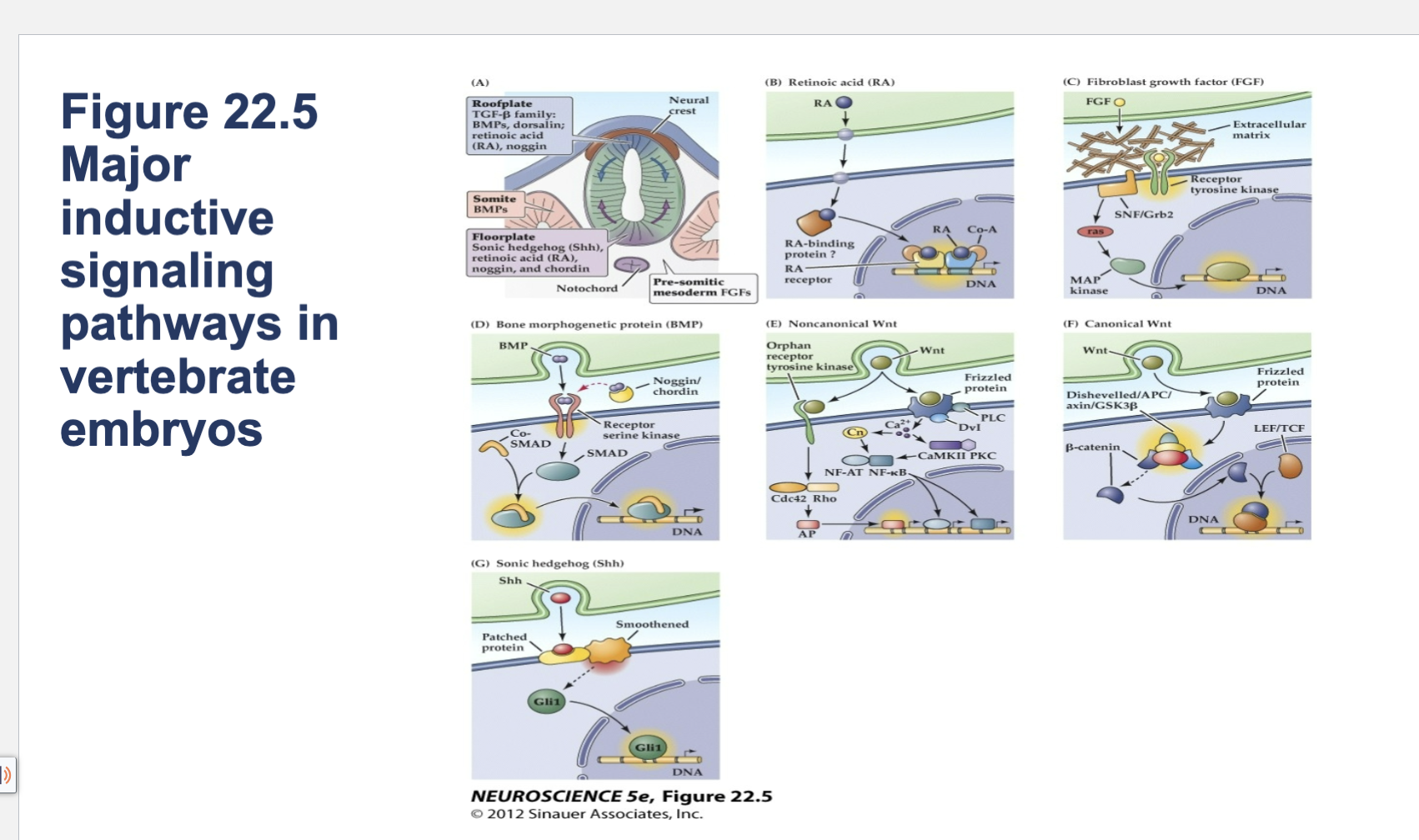
Figure 22.5- Major inductive signaling pathways in vertebrate embryos.
A) The embryonic notochord, floorplate, and neural ectoderm, as well as adjacent tissues such as somites, produce the molecular signals that induce cell and tissue differentiation in the vertebrate embryo. (B–G) Schematics of ligands, receptors, and primary intracellular signaling molecules for retinoic acid (RA); members of the FGF and TGF-â (BMP) superfamilies of peptide hormones; the Wnt family of signals; and Sonic hedgehog (Shh). Each of these pathways contributes to the initial establishment of the neural ectoderm, as well as to the subsequent differentiation of distinct classes of neurons and glia throughout the brain.
FGF vs Retanoic Acid
FGF receptor is sitting on the cell surface in membrane while retanoic acid’s receptor is not
Neurogenesis
The production of new neurons
Begins after the initial patterning of the brain is complete
Precursor cells are located in the…
Ventricular zone (innermost cell layer surrounding the lumen of neural tube)
Ventricular Zone
Innermost cell layer surrounding the lumen of the neural tube
All neurons are produced…
before birth
after birth precursors will disappear and no new neurons will be produced to replace those lost due to age, injury or disease
*****exceptions to this; adult
neurogenesis does occur
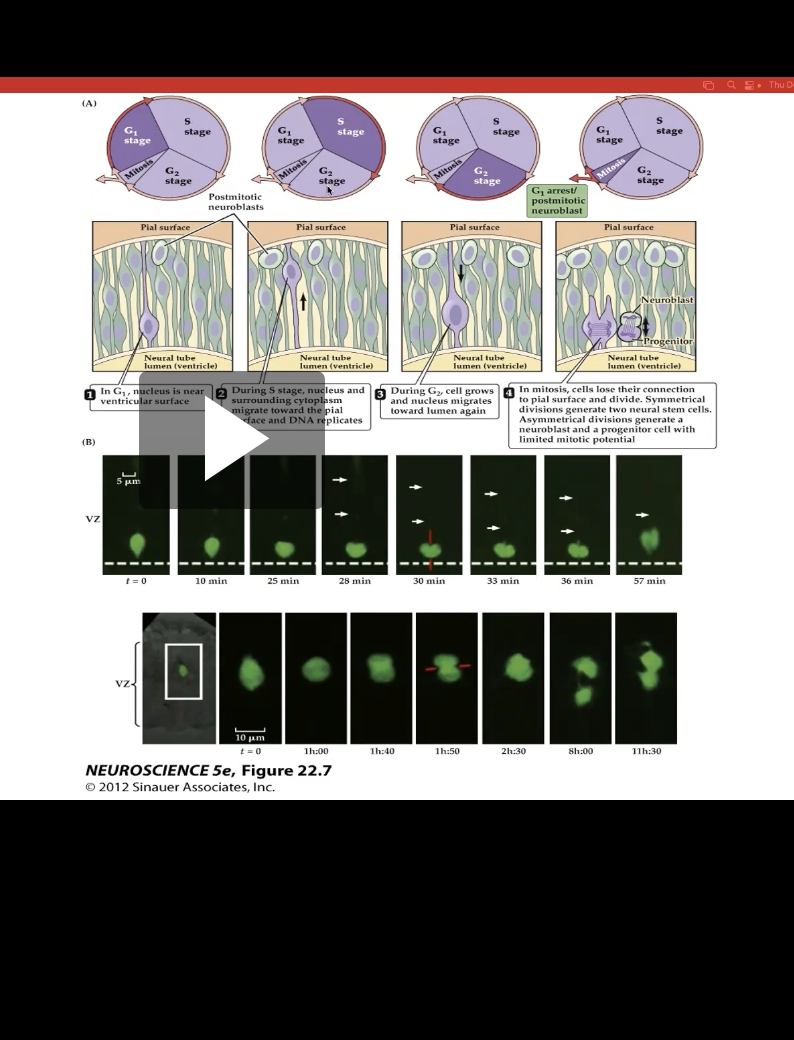
Figure 22.8- Neural precursor cells undergo mitosis in the ventricular zone.
Shows location change of proliferating cells
Ex: In first img, nucleus is near ventricular surface, meaning closer to cortex
As proliferating cells it moves through S phase it moves towards the cortex
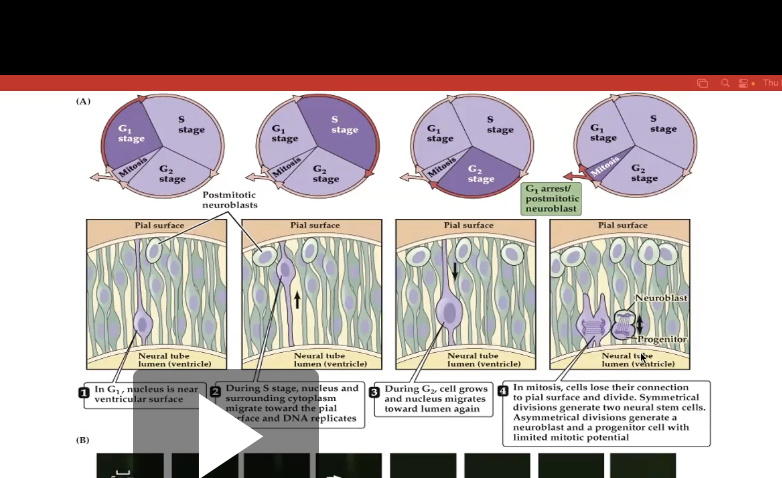
Fig 22.8- Symmetrical Division vs Asymmetrical Division
Symmertrical
- Produces 2 neural stem cells
Asymmetrical
- Produces 1 neural stem cell and 1 neuroblast that is a cell that differentiate from radial glial cells and are committed to becoming neurons (Shown in panel 4)
3 factors that Influence Differentiation of Neurons and Glial Cells
Neural Induction
Organizer Centers
Neural Patterning
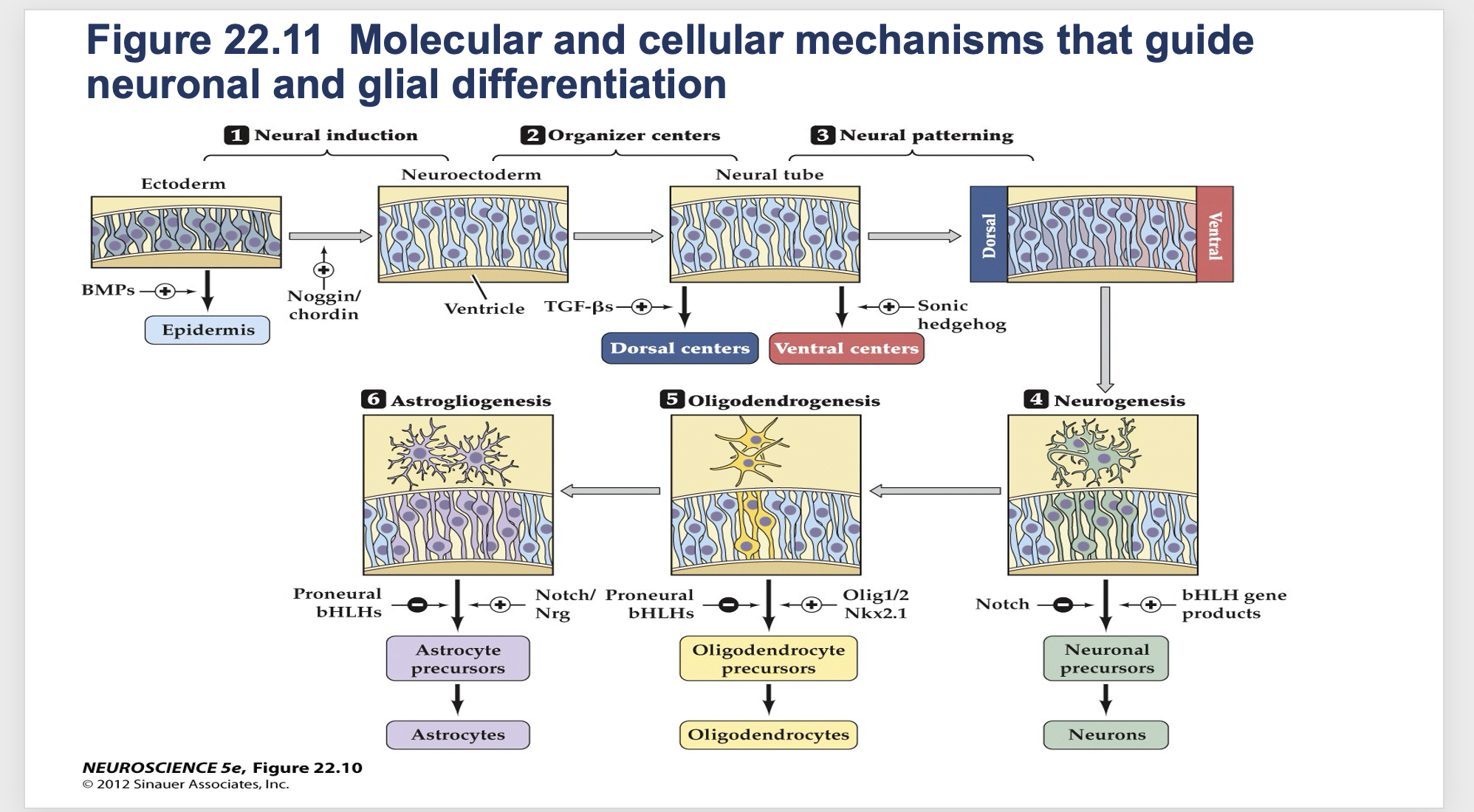
Figure 22.11- Molecular and cellular mechanisms that guide neuronal and glial differentiation.
Factors that influence differentiation of neurons and glial cells
Neural induction
-normal embryonic stem cell induced to have neural fate to become either neuron or glial cell
Organizer Centers
- Determines if cells should be in dorsal or ventral center
Neural Patterning
- The cell moves and migrates to right place
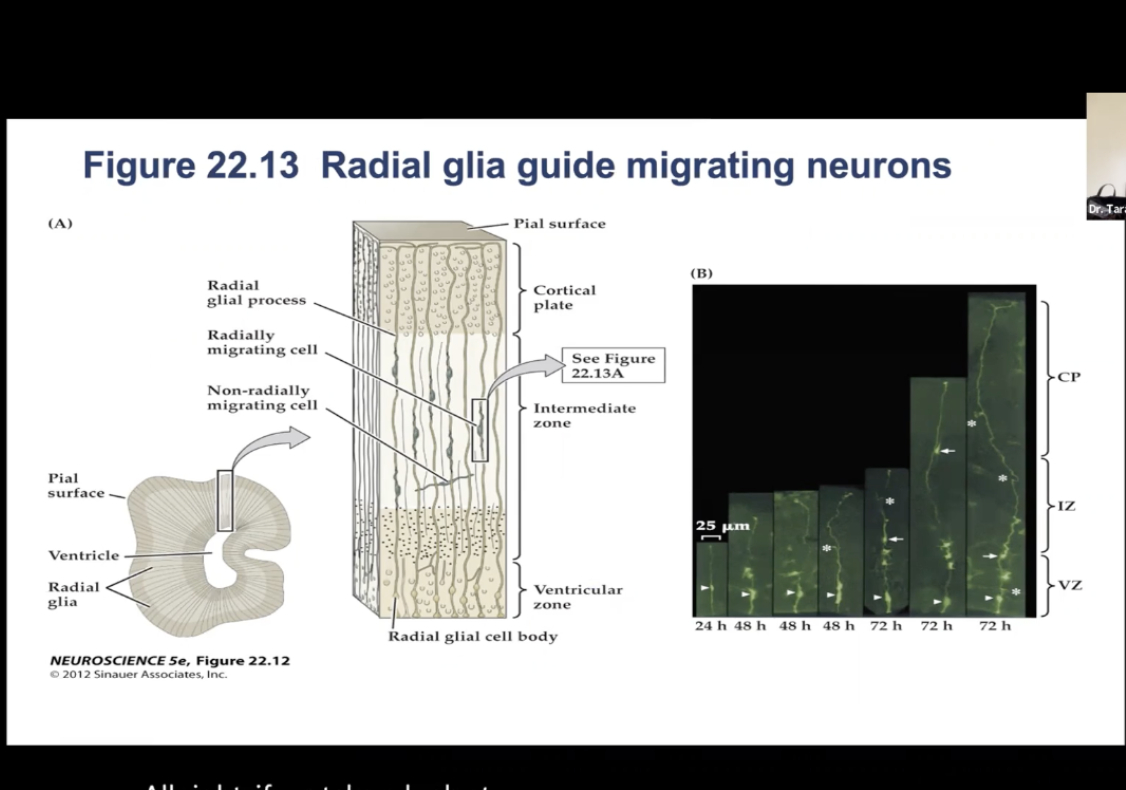
Migration of Neurons: Figure 22.13- Radial glia guide migrating neurons.
(A) Radial Glia provide framework for the neurons to migrate along to their final position in the cortex. Some cells take a nonradial migratory route, which can lead to wide dispersion of neurons derived from the same precursor.
(B) Time-lapse micrography showing radially migrating and nonradially migrating neurons.
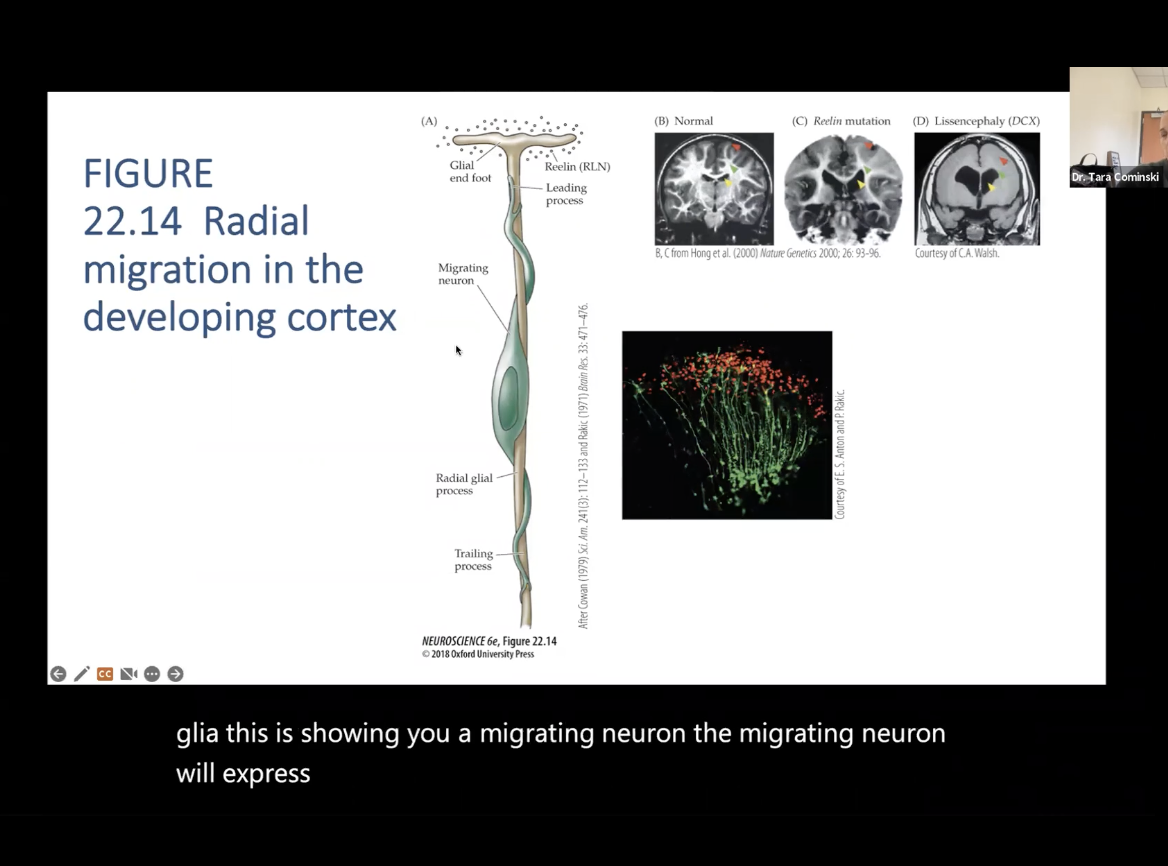
Migration of Neurons: Figure 22.14- Radial migration in the developing cortex
(A)- Shows what will happen if there is a mutation in any one of the genes involved in migration
Migrating neuron will express particular set of genes when it is migrating, so if there is a mutation, then can lead to disruption in brain development
B- Normal Brain
C- Less cortical and less white matter tracts, enlargement of ventrical
D- Smooth brain, lack of brain tissues, loss of white matter tracts
Spina Bifida (spinal cord does not develop normally)
failure of the neural tube to close completely – insufficient intake of folic acid
Anencephaly (missing parts of the brain and skull)
failure of the neural tube to close at all
Exogenous exposure to retinoids (teratogens)
Accutane (acne treatment, 1980’s) – increase in spontaneous abortions and birth defects
Retinoic acid – important for proper development of the nervous system; alters gene expression of SHH (Sonic Hedgehog)
Holoprosencephaly (Mutations in human genes for SHH or related receptors are associated with serious disorders)
disrupted regional differentiation of the forebrain, improper development of cerebral hemispheres
1 in 16,000 live births and most common malformation of the forebrain known
Cyclopia – development of a single eye
Range of severity from mild to still birth – responsible for 1 in 250 still births
Medulloblastoma (Mutations in human genes for SHH or related receptors are associated with serious disorders)
Result of cancerous transformation of cerebellar granule neuron precursors
Most common childhood brain tumor; 60 % survival rate
Rare – between 1 in 50,000/100,000 births
Basal cell carcinoma (Mutations in human genes for SHH or related receptors are associated with serious disorders)
Most prevalent form of skin cancer
What is cell proliferation in neural development?
Is the process by which neural precursor (stem) cells divide to produce more precursor cells.
This expansion of the cell population is essential to ensure there are enough cells to form the different parts of the nervous system.
What is cell migration in neural development?
Is the movement of newly formed neurons and glia from their origin (typically in the ventricular zone) to their final destinations in the developing nervous system. Radial glia often guide this movement, especially in the cortex.
What is cell differentiation in neural development?
Is the process by which neural precursor cells develop into specific types of neural cells—such as neurons, astrocytes, or oligodendrocytes—based on molecular signals received from their environment (e.g., floorplate, roofplate, notochord).