Lecture 24 Neuropeptides and gaseous neurotransmitters
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
Type 1 and 2 neurotransmitters vs. neuropeptides
Type 1 and 2: classical neurotransmitters- monoamines, amino acids. etc
precursor molecules before neurotransmitter (memorize those)
synthesis happening in the axon terminal of the neuron- Where NT is close to the small clear vesicles they are transported in
vesicles: small, clear
Neuropeptides
precursor molecules are synthesized at the cell body
transported down axon to axon terminal
processing of the protein into neuropeptide takes place in vesicle as its being transported down the axon
large, dense core vesicles
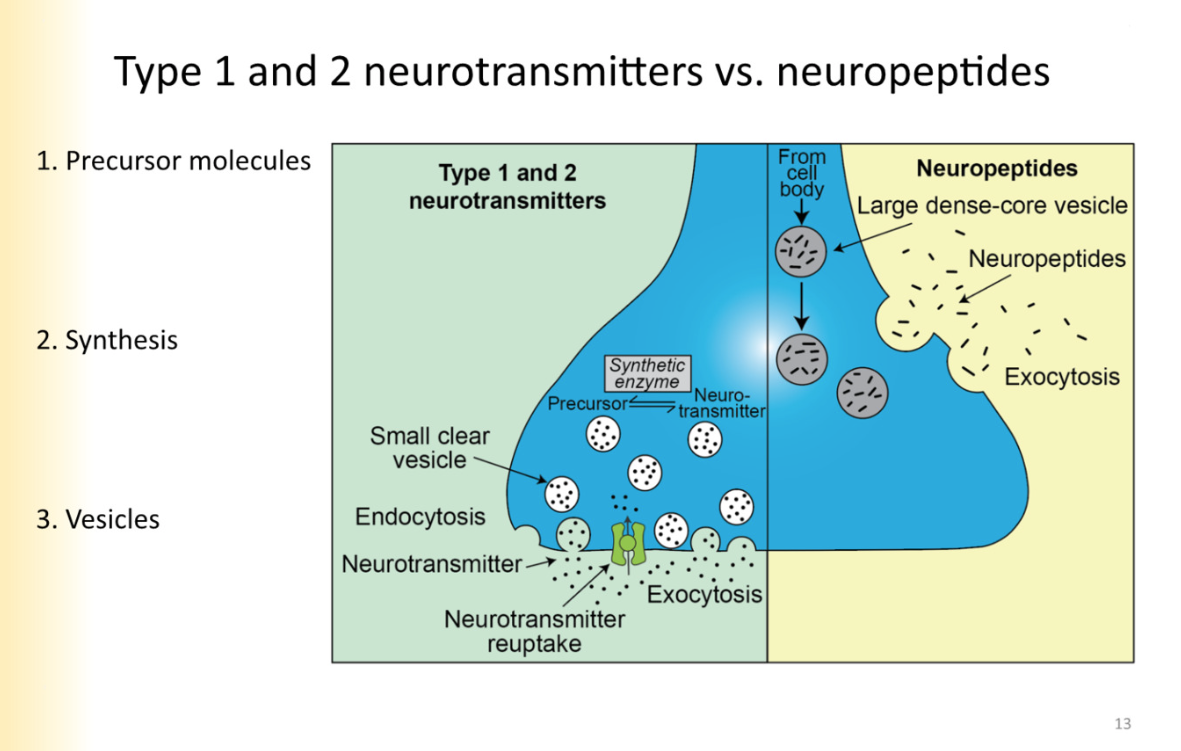
Compare and contrast Type 1 and 2 vesicles vs neuropeptides
small clear:
released from active zone
primed at active zone
large, dense core
bigger
contain neuropeptides
released extrasynaptically
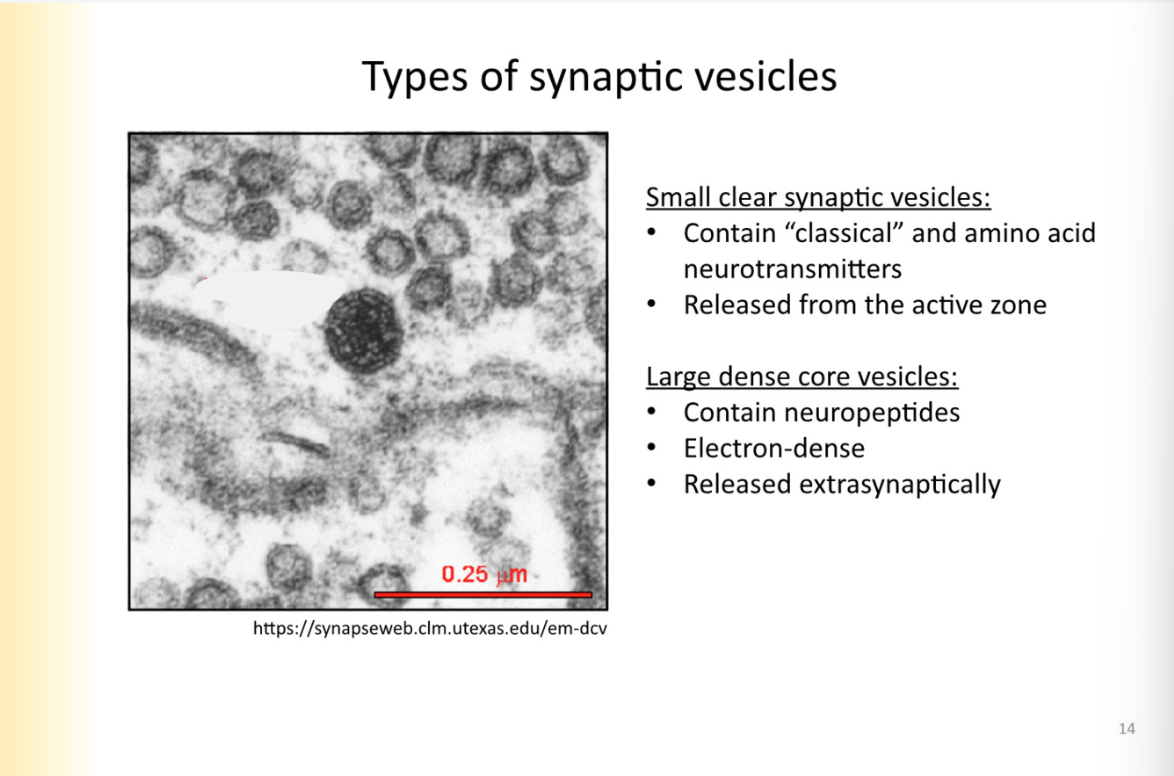
Neuropeptide synthesis
protein that is precursor- synthesized in cell body
nucleus, ER, golgi (protein) -
protein in vesicle is called: prepropeptide
pre- signal sequence (not a part of final product)
pro: peptide is NOT complete yet
peptide: final product
vesicle buds off golgi and becomes transport vesicle to the axon terminal where transport vesicle is now dense core vesicle
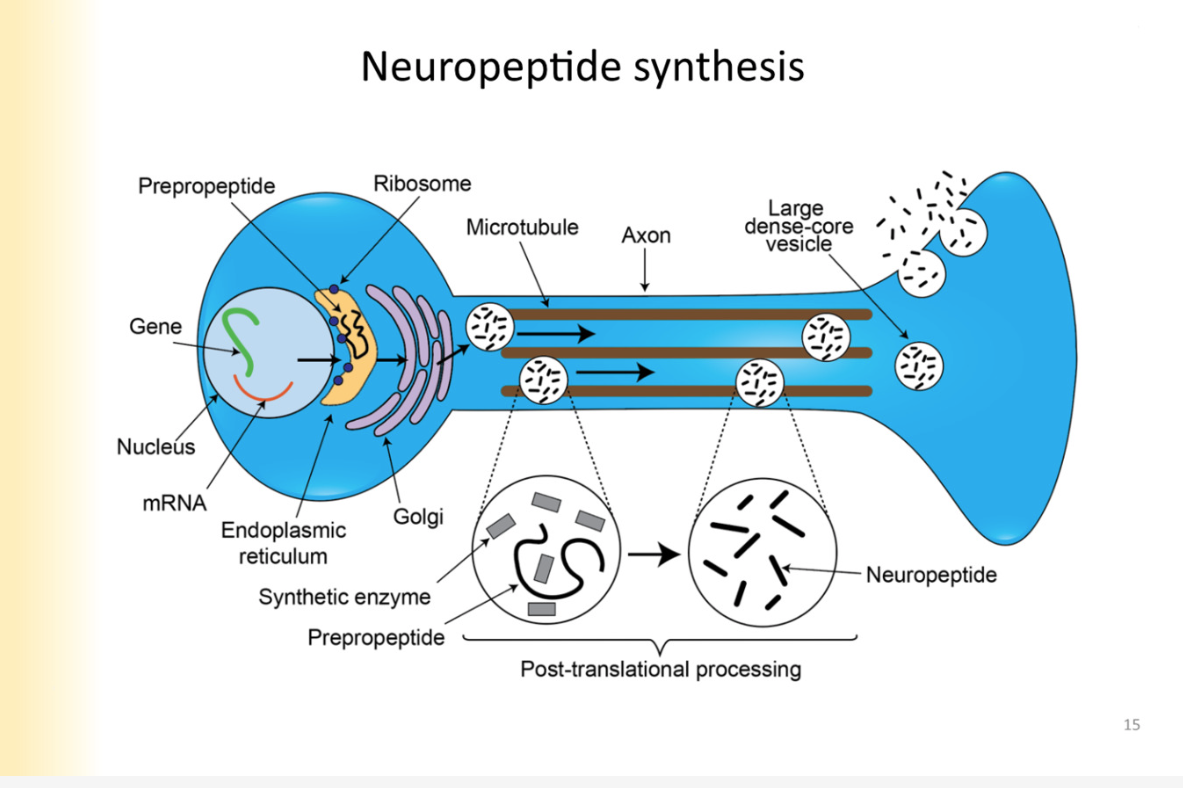
Post-translational processing of neuropeptide
*happening inside vesicle as it is transported down the axon
inside transport vesicle— there must be synthetic enzymes to process propeptide to final
Diversity in neuropeptides created here:
EXMAPLE:
precursor pepride: POMC
depending on enzymes that are put in vesicle with the POMC, the POMC can be converted to a variety of products (ACTH or b-LPH)
Those peptides can be further processed by additional enzymes based on end product- (ex. in the intermediate lobe of a gland)
further diversity is possible, converted into different compounds
One precursor with a lot of final signalling neuropeptides
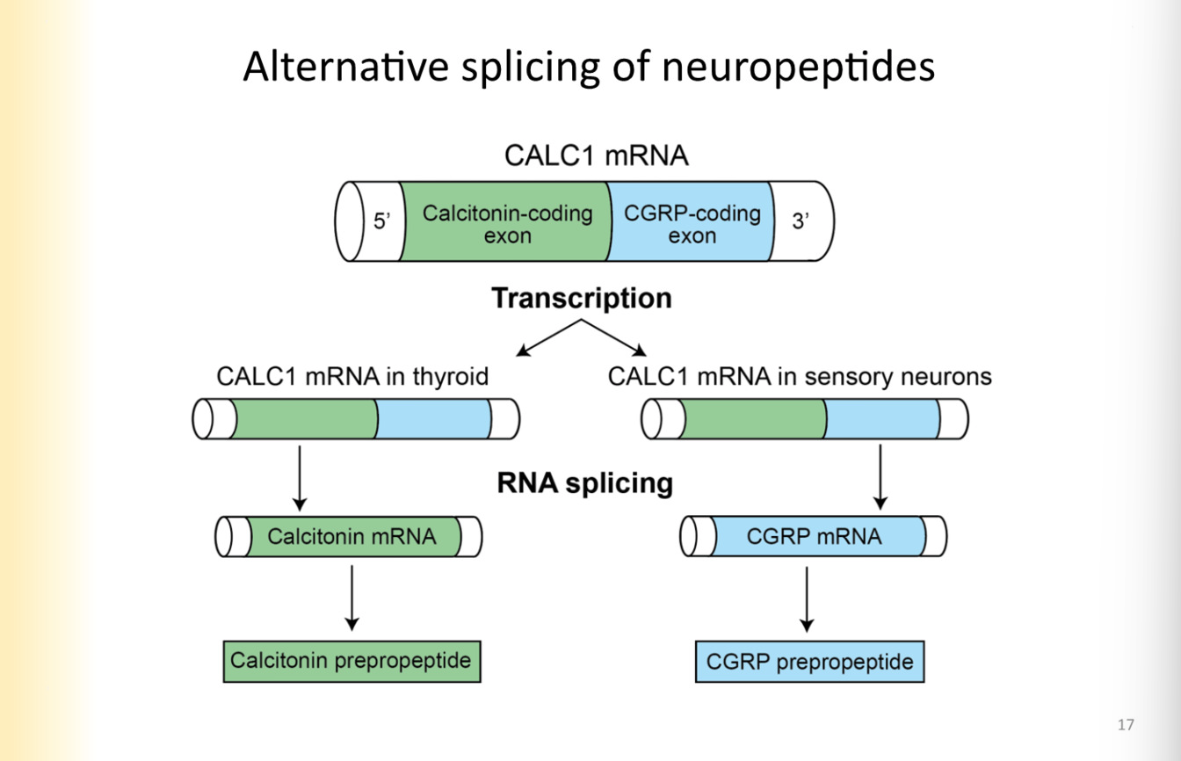
Alternative splicing of neuropeptides
Happening at mRNA level- pre-translation
different coding regions in the same gene can code for different neuropeptides
they can be spliced to be separated
example:
CALC 1 MRNA is spliced to have calcitonin coding region and cGRP mRNA region
means a smaller portion of genes can give rise to more neuropeptides
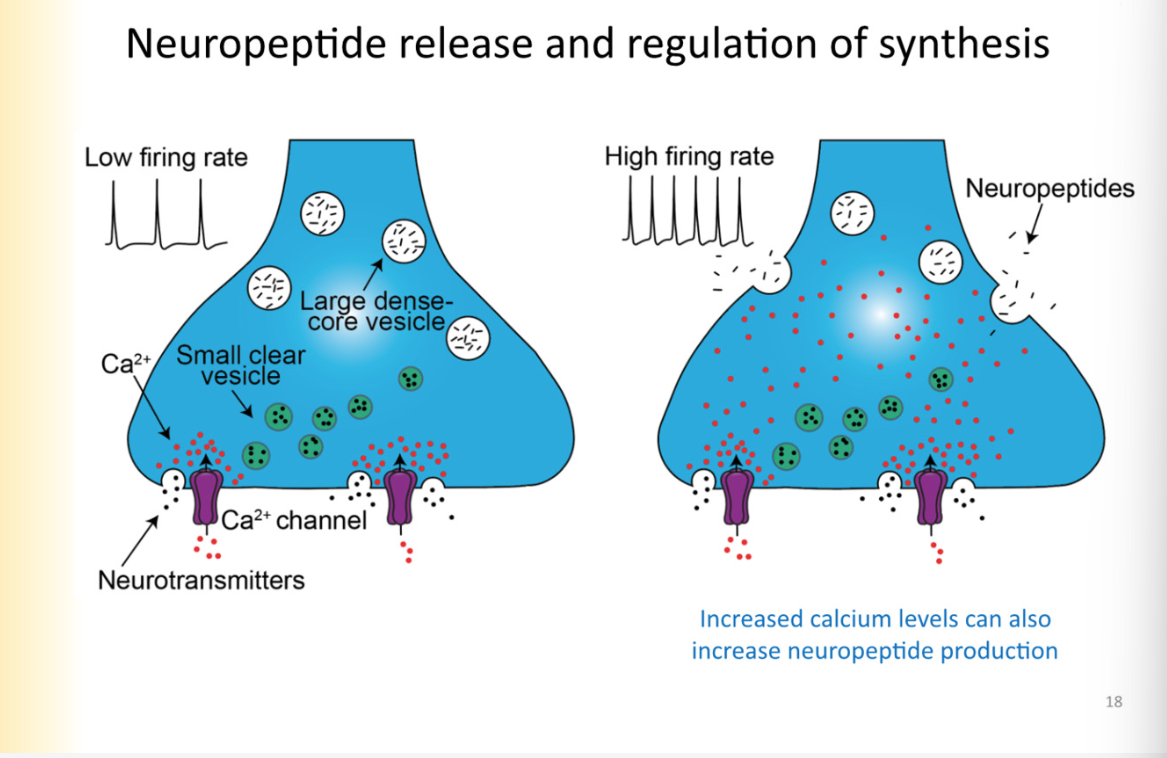
Neuropeptide release and regulation of synthesis (ca2+ relationship and production of neuropeptide)
Dense core vesicles are not docked at active zone but instead released extrasynaptically
require higher concentrations of ca2+ in the terminal to trigger NP’s to be released
when there is low frequency activity- the ca2+ released in the presynaptic cell is quickly taken up by buffers and etc
it is not able to travel through the axon terminal
need high enough concentration that it can easily saturate buffers and diffuse to extra active zone locations of large dense core vesicles and trigger them to be released in areas outside of the active zone
this comes from high firing rate
ALSO:
increased ca2+ levels can also increase neuropeptide PRODUCTION
high frequency activity is when dense core vesicles will be released, increase synthesis of peptides
to produce more neuropeptide, signal has to get back to SOMA
Concentrations and binding affinities for neuropeptides vs Type 1 and 2 neurotransmitters
Type 1 and 2:
high concentration of vesicle released
acting right at the synapse, across from active zone
Neuropeptides:
always neuromodulators
always act on metabotropic receptors
metabotropic receptors have high binding affinity, therefore, the receptor will be activated with lower concentrations of it (low kd)
even though they are larger vesicles, less vesicles are released, therefore NP is in lower concentration because receptors have higher binding affinity
Neuropeptide termination/degradation
No, there is no reuptake/recycling of neuropeptides
enzymatic degradation happens through peptadases
Neuropeptides are critical for function
NPY receptor activated, targets GIRK channel:
Feeding behavior
homeostasis
circadian rhythm
stress and anxiety- increased levels of NPY affect anxiety and stress
Gaseous neurotransmitters
Nitric oxide- vasodilation
Carbon monoxide
hydrogen sulfide
they are free radical compounds thought to contribute to neurodegeneration
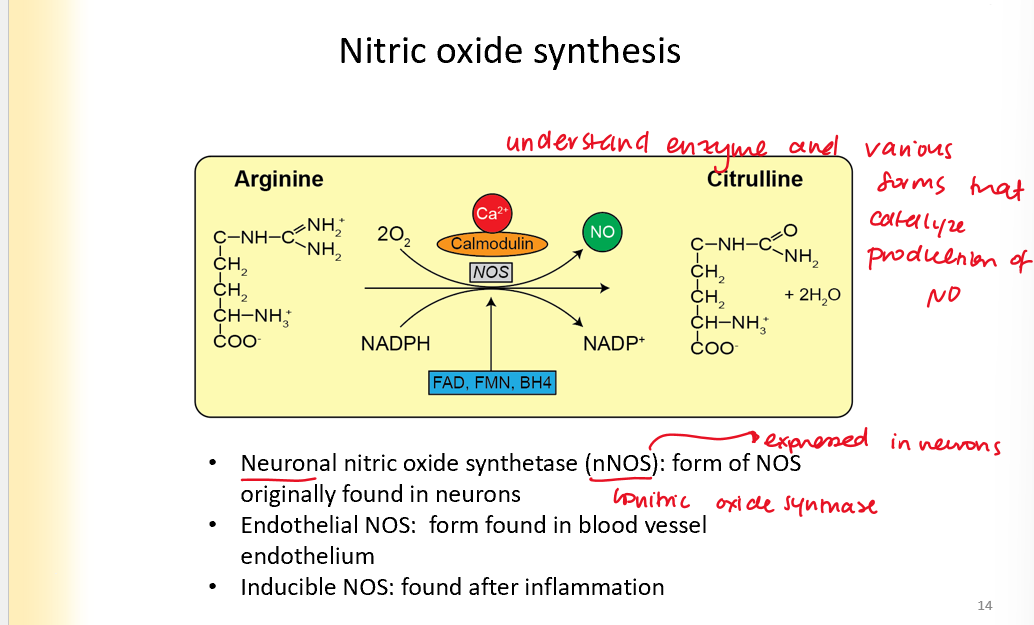
Nitric oxide synthesis
Is produced in the process of arginine turning into citruline
enzyme: nitric oxide synthetase
Neuronal NOS: found in neurons
endothelial NOS- blood vessel endothelium
Inducible NOS: found after inflammation
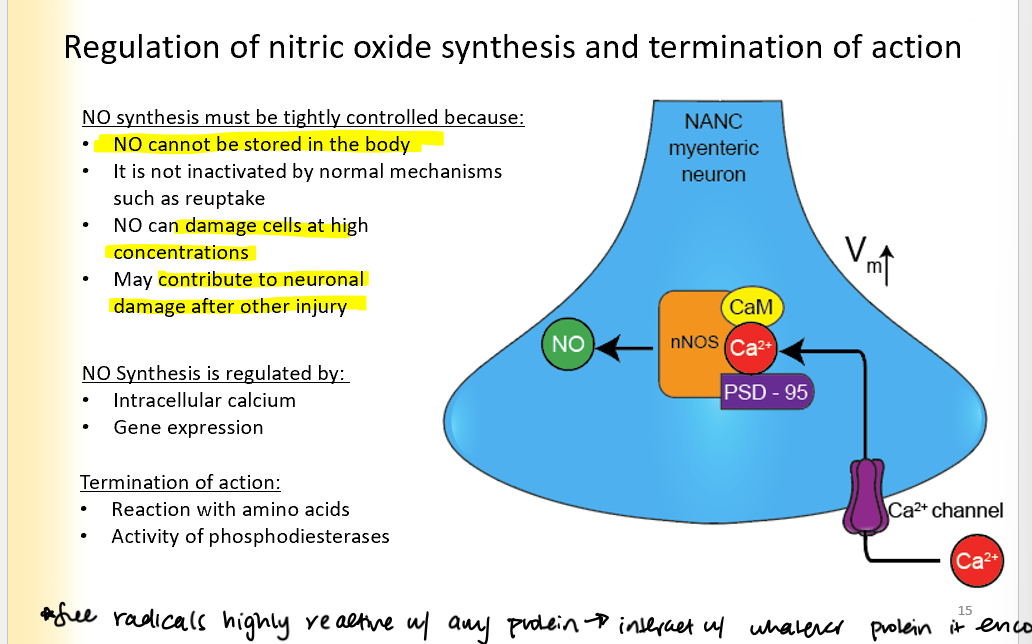
Regulation of Nitric oxide synthesis and termination of action
NO synthesis must be tightly controlled because:
NO cannot be stored in the body
It is not inactivated by normal mechanisms such as reuptake
NO can damage cells at high concentrations
May contribute to neuronal damage after other injuries
Regulated by:
Intracellular ca2+ (ca2+ activates the channel)
gene expression
Termination of action:
reaction with amino acids
activity of phosphodiesterases
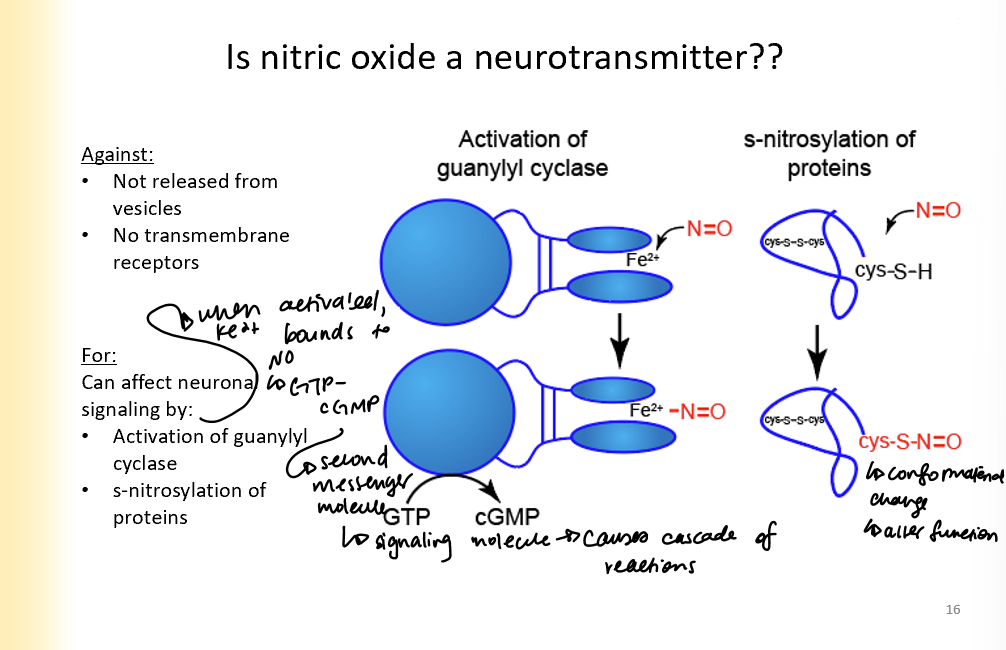
Arguments for and against Nitric oxide as a neurotransmitter
Against:
not released from vesicles
no transmembrane receptors
For:
can affect neuronal signaling by:
activation of guanylyl cyclase (Fe2+ binds to NO, then gmp turns into cGMP which is second messenger and causes signalling cascade)
s-nitrosylation of proteins (causes conformational change that alters function)
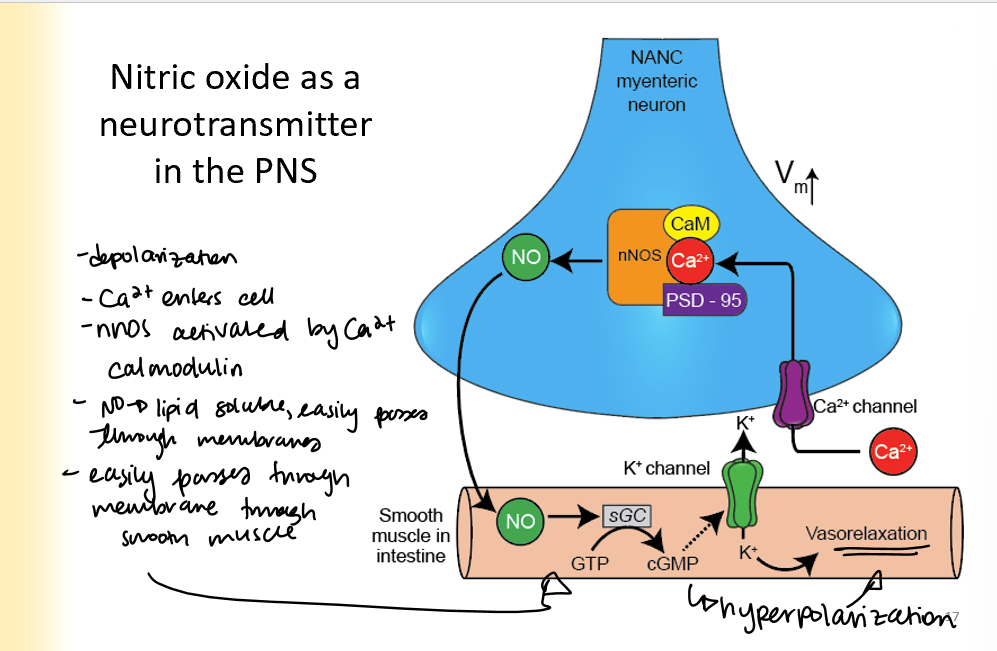
Nitric oxide as a neurotransmitter in the PNS
presynaptic cell becomes depolarized
Ca2+ enters the cell
nNOS activated by Ca2+ and calmodulin
NO is lipid soluble and easily passed through membranes
goes to smooth muscle, turns GTP into cGMP and causes vasorelaxation
NO as a neurotransmitter in CNS
1-2% of CNS neurons have NOS
nNOS activated in the post-synaptic terminal by ca2+ that NMDA passes through
can travel to adjacent neurons and presynaptic neurons
effects of NOS:
neurotransmitter release
synaptogenesis
apoptosis
synaptic plasticity
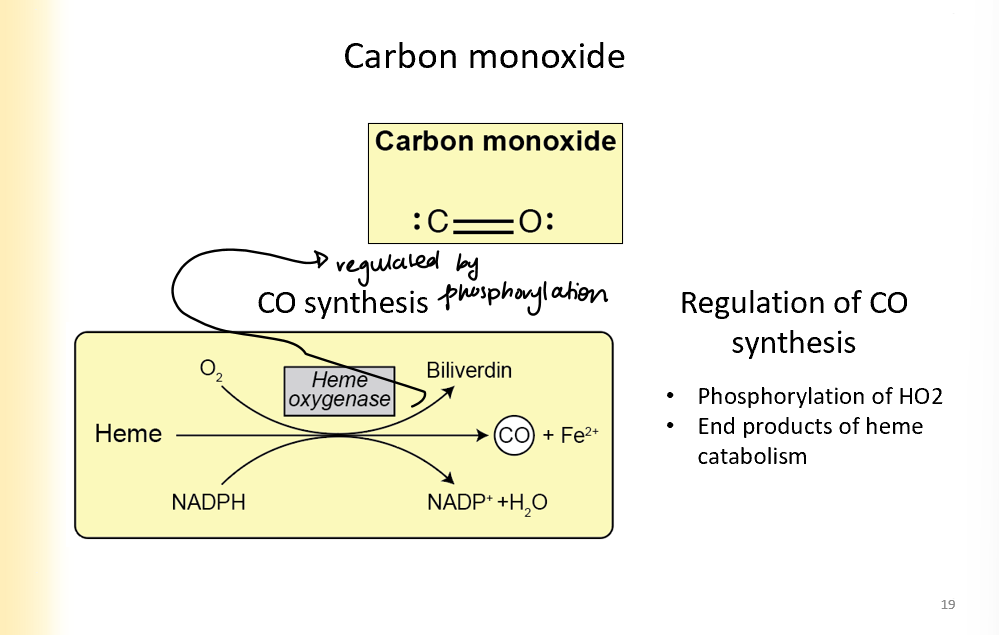
Carbon Monoxide SYNTHESIS
Iron and CO are the end products of heme catabolism
Heme goes through heme oxygenase to make Co and Fe
CO2 as neurotransmitter in PNS
ca2+ comes to presynapse
activates PKC
PKC activates HO2
creates CO
CO goes to smooth muscle in intestine and causes vasorelaxation
GTP to cGMP activates K+ channels (hyperpolarization)
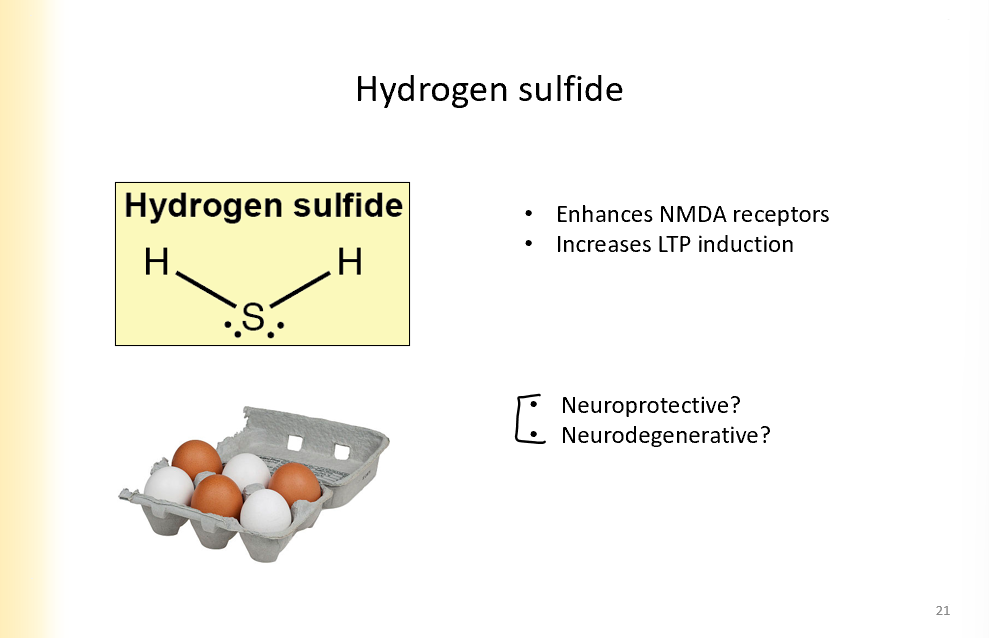
Hydrogen sulfide
enhances NMDA receptors
increases LTP induction
dont know if it is neuroprotective or degenerative yet
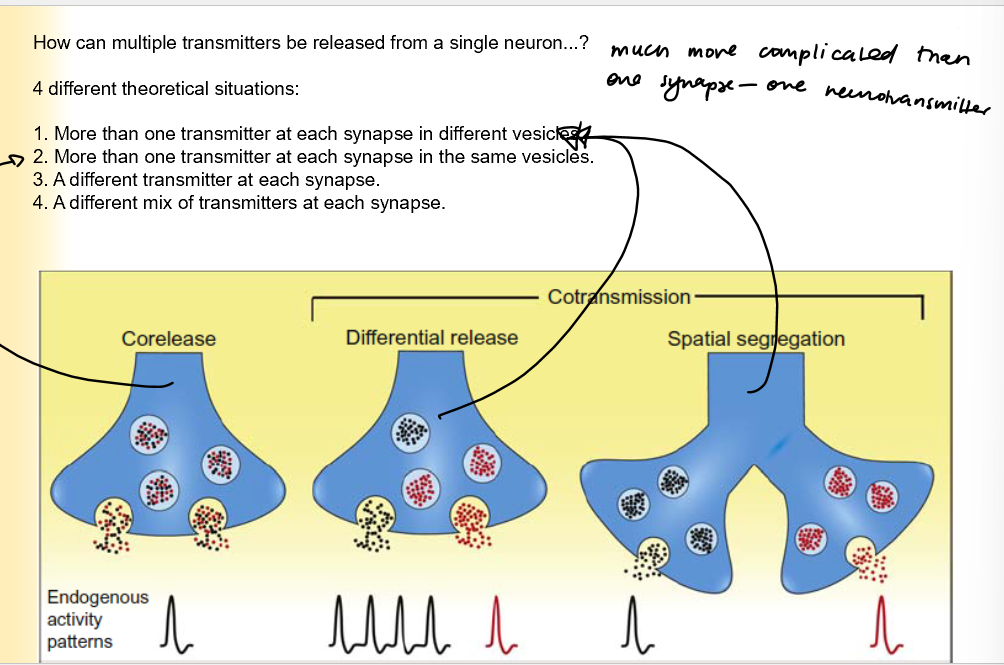
Scenarios where multiple transmitters are released from a single neuron
More than one NT at each synapse in different vesicles
More than one transmitter at each synapse in the same vesicles
A different transmitter at each synapse
a mix of NT at each synapse
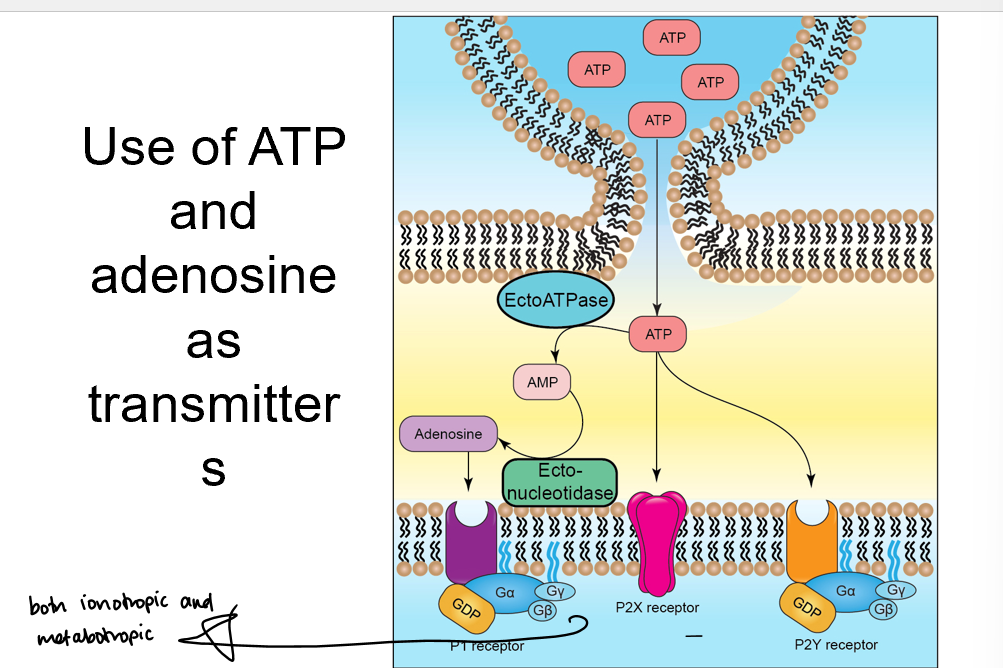
Purine transmitters
ATP is broken down into adenosine by ectoATPase and ectonucleotidease
adenosine receptors on membrane
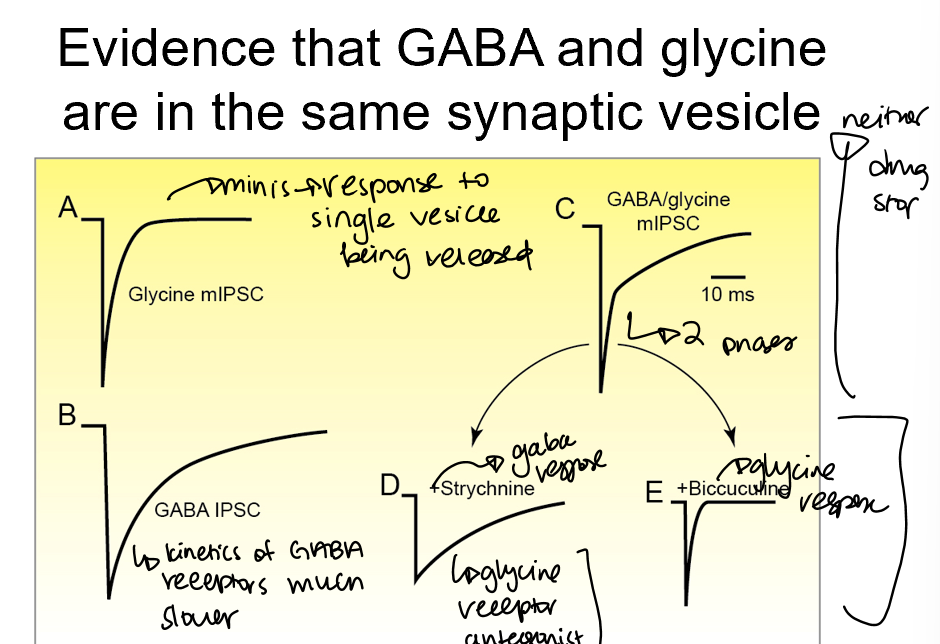
Evidence that GABA and Glycine are released from the same vesicle
when you apply GABA receptor antagonists (biccuculine), you just get glycine fast current
When you apply glycine receptor antagonists (strychnine), you get slow release GABA
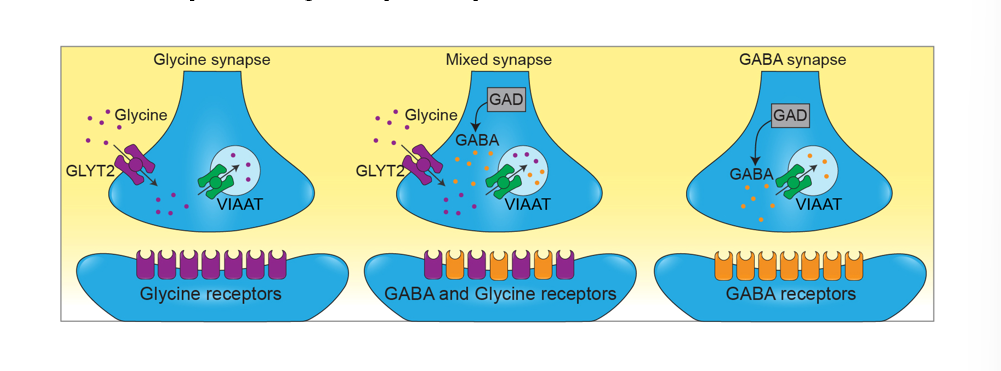
Nerve terminals in the spinal cord can release GABA and Glycine by expressing the required pre and postsynaptic proteins for both
refer to pic
transporters for glycine and the precursor for GABA are both present in mixed synapses that has both of them in vesicles present
Can the golgi preferentially sort vesicles
yes
golgi can send different transmitter containing vesicles to different axon collaterals at different synapses