C2.4 Ions and ionic bonds
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
6 Terms
What is an ion?
An ion is an atom or a molecule which has a positive or negative charge due to the loss or gain of electrons
They lose or gain electrons to get a full outer shell
What is an ionic bond?
An ionic bond is the electrostatic force of attraction between oppositely charged ions (anions and cations) formed by the transfer of electrons from a metal atom to a non-metal atom
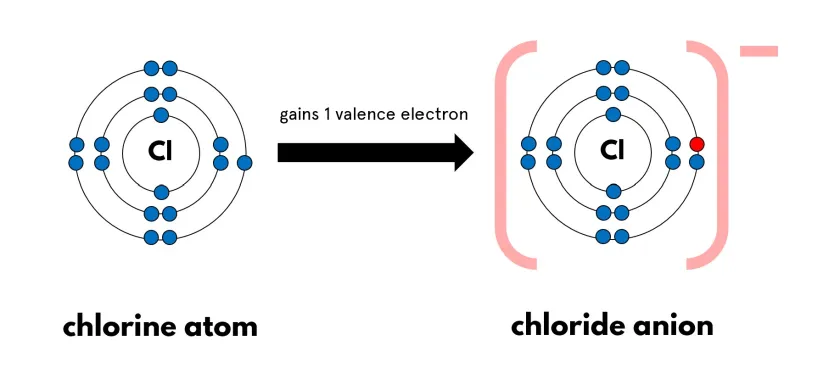
What is the name of a negative ion?
Anion
Gain electrons
Negative charge
More electrons than protons
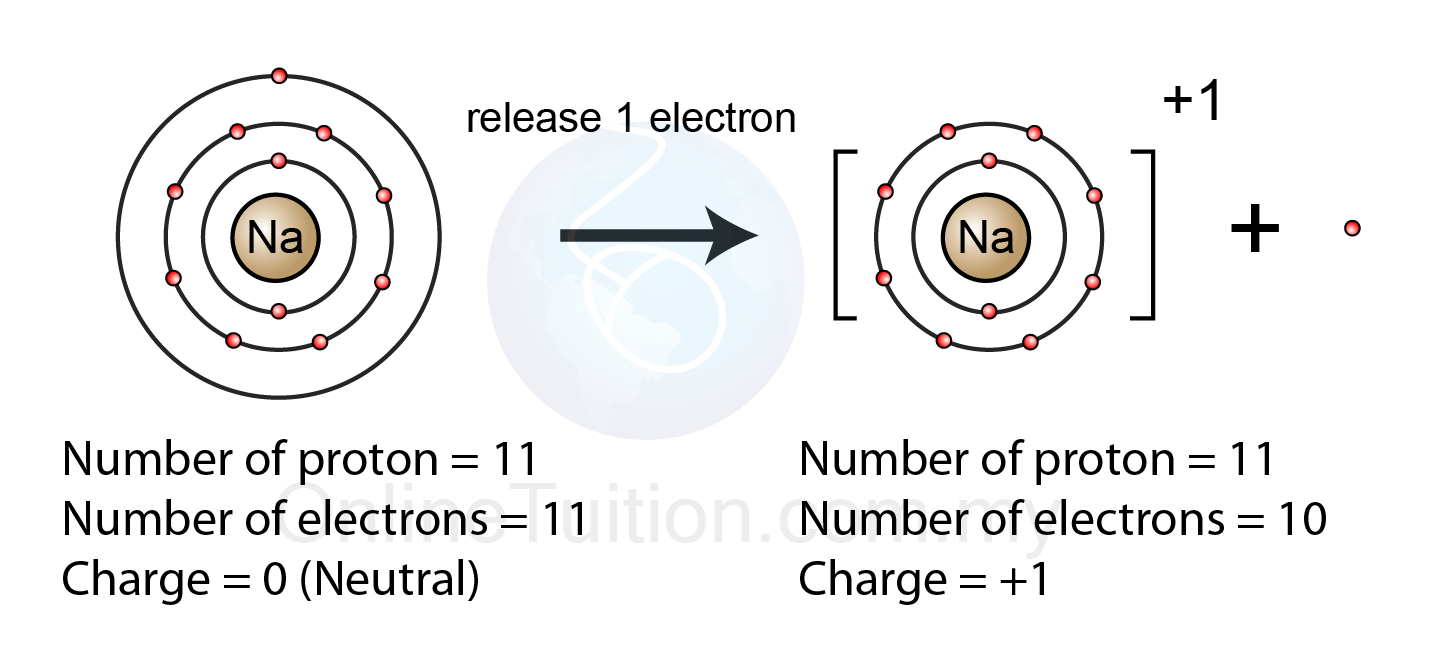
What is the name of a positive ion?
Cation
Lose electrons
Positive charge
More protons than electrons
What are the properties of ionic compounds?
High melting and boiling points: they have strong electrostatic forces of attraction (acting in all directions) that require lots of energy to break so they have high melting and boiling points
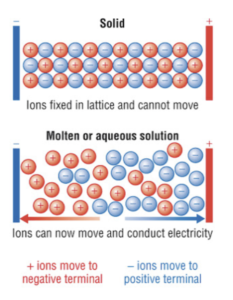
Good electrical conductivity when molten or aqueous and poor when solid: ions in the ionic lattice are usually unable to move since they are held together by strong electrostatic forces of attraction. When the ionic compounds is molten or dissolved in water, these ions are able to move freely and carry a charge
Generally soluble in water
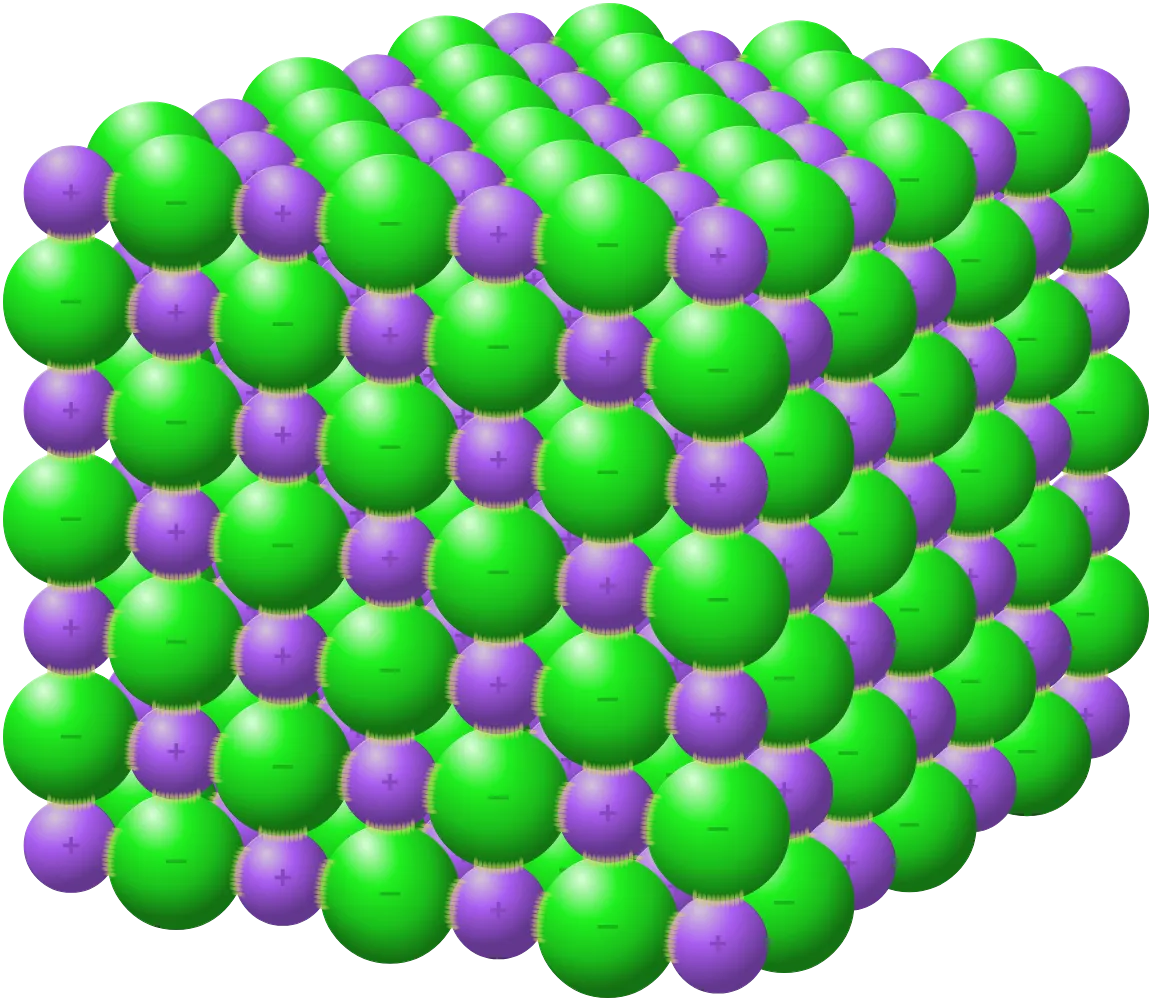
Describe the structure of an ionic lattice
Structure made up of positively charged ions (cations) and negatively charged ions (anions)
Packed together in an ordered, repeating pattern
Alternating ions (negative ions next to positive ions and so on)
Strong electrostatic forces of attraction that take a lot of energy to break