Experiment 5: Reductive Amination (Pt. 2)
0.0(0)
0.0(0)
Card Sorting
1/13
Earn XP
Last updated 7:43 PM on 6/8/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
1
New cards

Ethanol - Is the highlighted atom best described as a hydrogen, proton, or hydride?
Proton
2
New cards

LiAlH4 - Is the highlighted atom best described as a hydrogen, proton, or hydride?
Hydride
3
New cards

Acetic acid - Is the highlighted atom best described as a hydrogen, proton, or hydride?
Proton
4
New cards

Methyl acetate - Is the highlighted atom best described as a hydrogen, proton, or hydride?
Proton
5
New cards

Benzene - Is the highlighted atom best described as a hydrogen, proton, or hydride?
Hydrogen
6
New cards

Pyridinium - Is the highlighted atom best described as a hydrogen, proton, or hydride?
Proton
7
New cards

Wilkinson's catalyst - Is the highlighted atom best described as a hydrogen, proton, or hydride?
Hydride
8
New cards
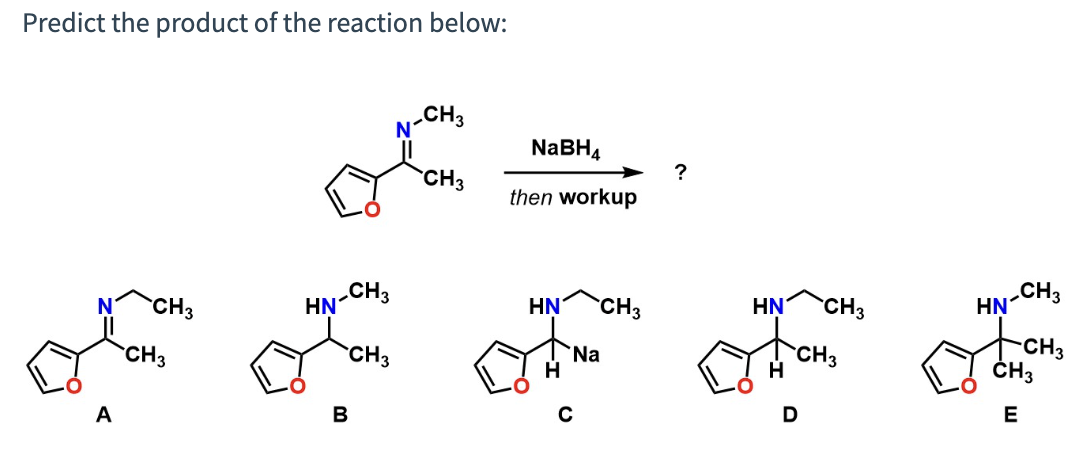
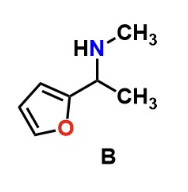
Predict the product of the reaction below:
B
9
New cards
What is the purpose of saving some imine for TLC analysis? Select all that apply.
To determine reaction progress and to compare Rf’s of the starting material and product
10
New cards
What is this gas likely to be?
Hydrogen gas
11
New cards
What is the aqueous layer?
\n Water and byproducts from NaBH4
12
New cards
\
What is the consequence if this step is omitted?
What is the consequence if this step is omitted?
\n There will be more water in the organic layer and will require more sodium sulfate for drying
13
New cards
What is the problem with having some ethyl acetate in the IR spectrum?
The signals from residual aldehyde starting material may be blocked
14
New cards
A TLC analysis using 10% EtOAc in hexanes indicated an Rf = 0.05 for the product. What should you do from here?
\
* Repeat the TLC analysis using a solvent mixture with greater polarity (50% EtOAc in hexanes) to increase the Rf to 0.3.
* Repeat the TLC analysis using a solvent mixture with greater polarity (50% EtOAc in hexanes) to increase the Rf to 0.3.