BIOL10008: Life and Macromolecules
1/26
Earn XP
Description and Tags
Concepts 1 and 2
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
Define the characteristics of living organisms.
made up of a set of common elements
Comprised of cells
Contain genetic info (allows coding of proteins)
Grow and change
Respond to environment
Use molecules + make new molecules
Extract + use energy
Exist in populations, can evolve
What evidence does DNA provide to explain evolution?
All organisms have the same genetic code (DNA or RNA) as they have all arisen from earlier primitive forms over the past 4 billion years through processes of evolution
Explain different hypotheses for life formation on Earth.
Chemical evolution - conditions on primitive Earth led to the formation of simple molecules, which led to the formation of life forms
Evidence - Miller–Urey experiment showed that it was possible for amino acids to be formed from gases that were hypothesized to have been in Earth’s early atmosphere
Extra terrestrial origin
Evidence - meteorite from space carried molecules that are characteristic of life on Earth
Discuss how Stromatolites provide evidence for the beginning of life on Earth.
Stromatolites - geologic formations which consist of layers of limestone, calcium carbonate
Allowed for the fossilization of bacteria such as cyanobacteria because they can be trapped in between the layers
The bacteria found can be dated back to the beginning of life
Properties of Water
Water molecules are able to interact with each other via hydrogen bonding, providing a high heat capacity
Polar molecule - oxygen more electronegative and thus carries a partially negative charge compared to the hydrogen atoms which carry a partially positive charge
Relatively high melting + boiling point
High heat of vaporization
Undergo cohesion (between h2o) and adhesion (attraction to other molecules)
less dense as a solid than as a liquid
Surface tension - water molecules at the surface are hydrogen bonded to other water molecules below them
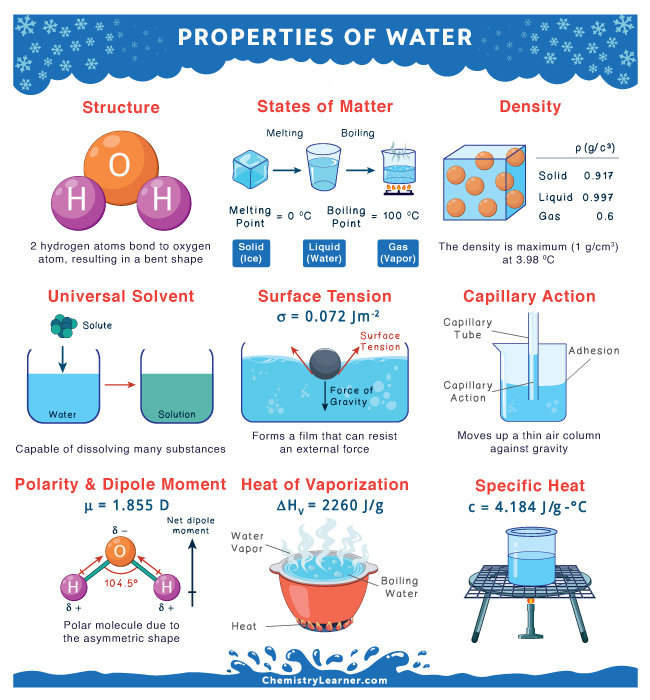
Describe how the properties of water are important for life
High heat capacity - takes a lot of energy to raise the temperature of a certain amount of water by a degree, so temperature can be regulated easily (stay constant)
High heat of vaporisation - heat is absorbed by water to break hydrogen bonds, allowing evaporation which has a cooling effect
Cohesive and adhesive properties - allow fluid transport
Solid form less dense than liquid - prevents ponds from freezing from the bottom up, allowing life to thrive under the insulating layer of ice
What is Dipole Dipole attraction
Exists between oppositely charged poles of two different molecules
Between polar molecules (have one or more polar bond)
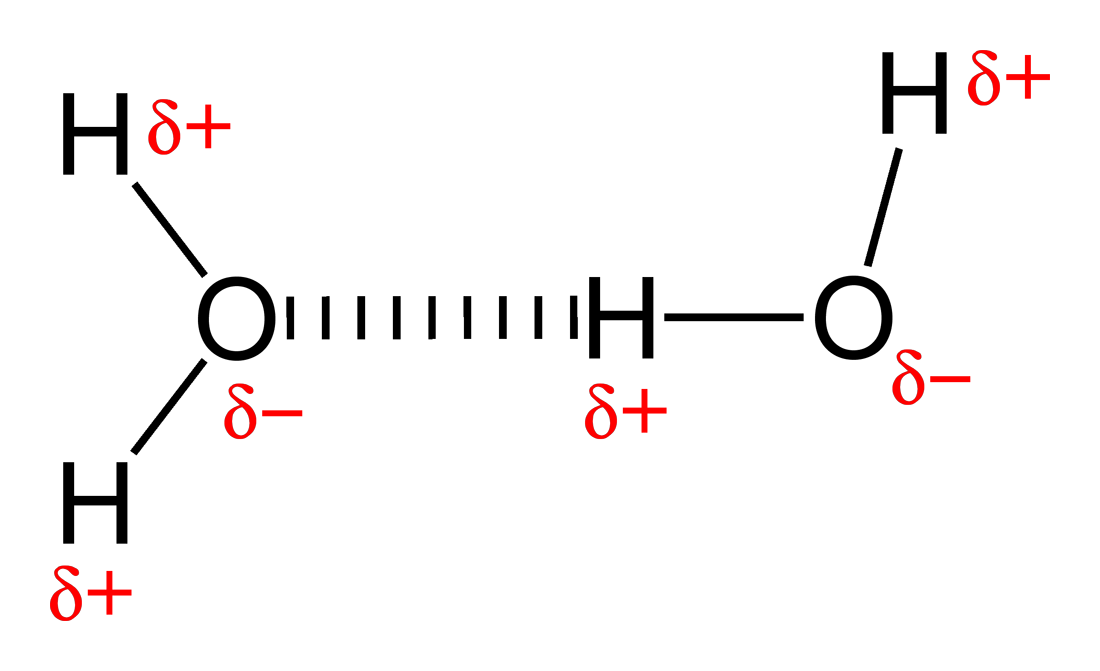
Describe a Hydrogen Bond
When hydrogen bonds to either F, O, or N, it becomes partially positively charged due to the difference in electronegativity. The H proton becomes exposed, and consequently is attracted to a lone pair of electrons on a different atom of any element.
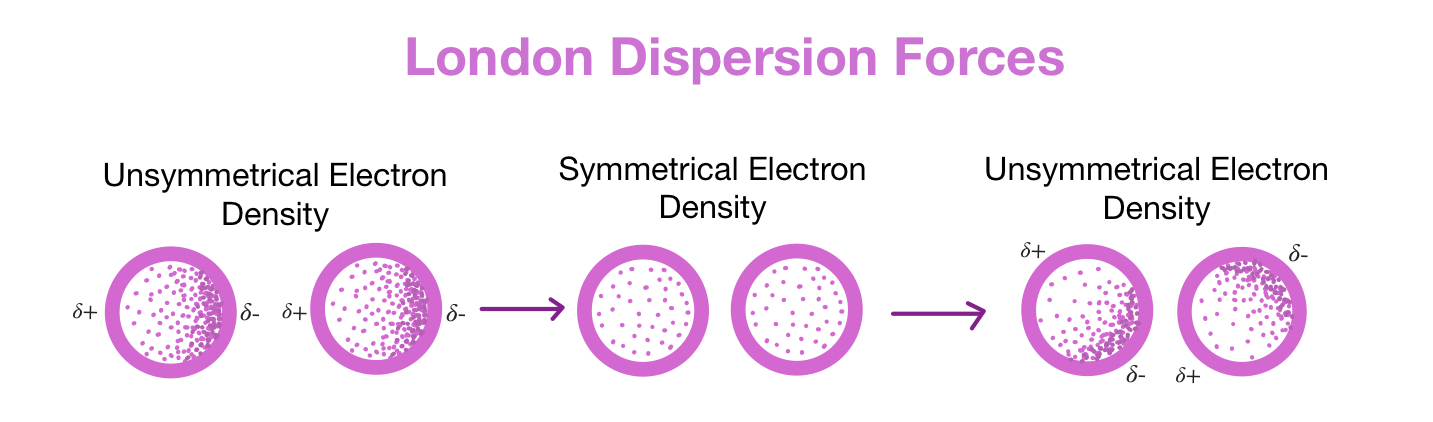
What are London dispersion forces?
Type of Van De Waals force
Present in any molecule, only force present between non-polar molecules
occur due to fluctuations in electron distribution that lead to momentary polarisation in one molecule, which induces polarization in another molecule and subsequent attraction between them
Bigger molecule = more electrons --> more frequent and stronger dispersion forces
What are the functions of carbohydrates in organisms?
General formula (CH2O)n
source of stored energy
used to transport stored energy
serve as carbon skeletons that can be rearranged to form new molecules
form extracellular assemblies such as cell walls that provide structure
How are carbohydrates categorised?
Monosaccharides (mono, “one,” + saccharide, “sugar”), such as glucose, are simple sugars. They are the monomers from which the larger carbohydrates are constructed.
Disaccharides (di, “two”) consist of two monosaccharides linked together. Through condensation reaction where water is lost and a glycosidic link (ether bond) is formed Ex: sucrose
Oligosaccharides (oligo, “several”) are made up of several (3–20) monosaccharides.
Polysaccharides (poly, “many”), such as starch, glycogen, and cellulose, are polymers made up of hundreds or thousands of monosaccharides.
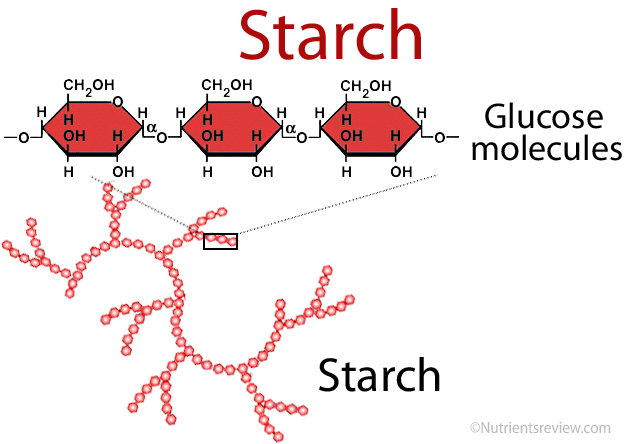
What is the role and structure of starch?
energy storage compound of plants
polymer of glucose
contain α-1,4 glycosidic bonds which produce branching at carbon 6
Branching limits the number of hydrogen bonds that can form in starch molecules, making starch less compact than cellulose, only to single molecule
readily hydrolysed into glucose monomers
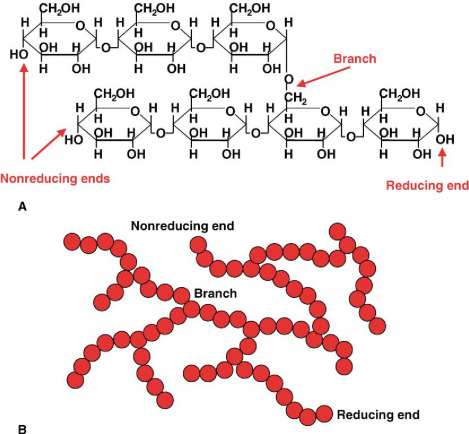
What is the role and structure of glycogen?
energy storage compound for animals
Polymer of glucose
Like starch, glycogen contains α-1,4 glycosidic bonds which produce branching at carbon 6
hydrogen bonding occurs between a single molecule
high amount of branching in glycogen makes its solid deposits more compact than starch
readily hydrolysed into glucose monomers
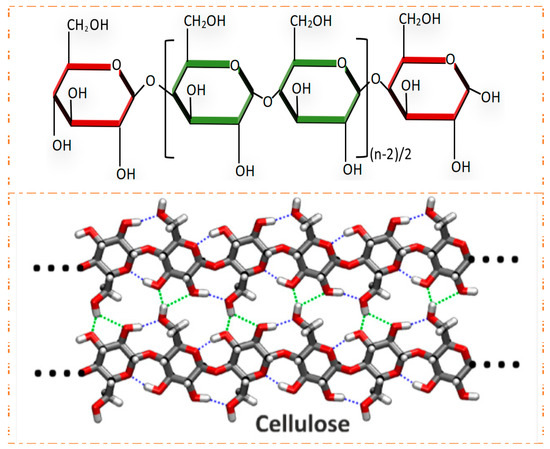
What is the role and structure of celluose?
component of plant cell walls
polymer of glucose
contain β-1,4 glycosidic bonds (-OH group facing up) that are chemically very stable - requires a different enzyme to hydrolyse compared to alpha glycosidic bonds
can form hydrogen bonds between adjacent molecules, resulting in bundles or fibres
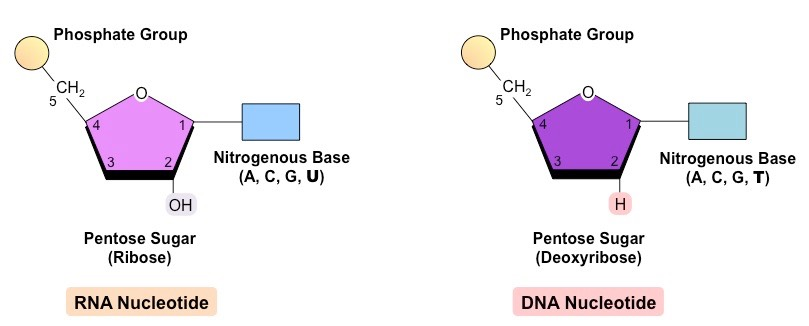
What is the structure of a nucleotide?
Composed of:
- Five-carbon pentose sugar (deoxyribose or ribose sugar)
- Phosphate group (attached to 5' carbon)
- Nitrogenous base (attached to 1' carbon)
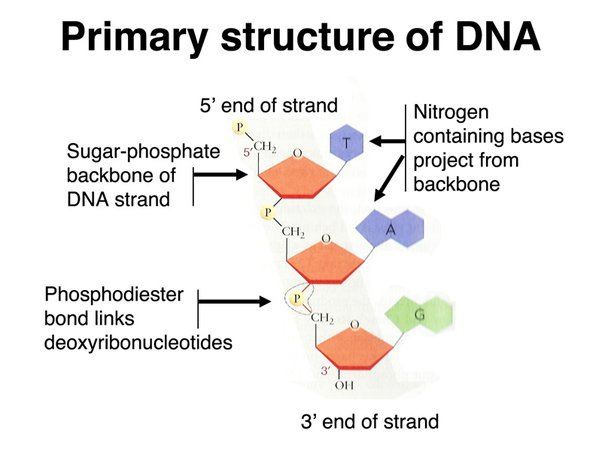
How are nucleotides linked together? How are DNA strands held together?
Hydroxyl from phosphate at 5' of one nucleotide reacts with hydroxyl on 3' carbon of another to form a phosphodiester bond
These bonds form the sugar-phosphate backbone of a nucleic acid (DNA or RNA)
Strands of DNA held by hydrogen bonds between complementary nitrogenous bases
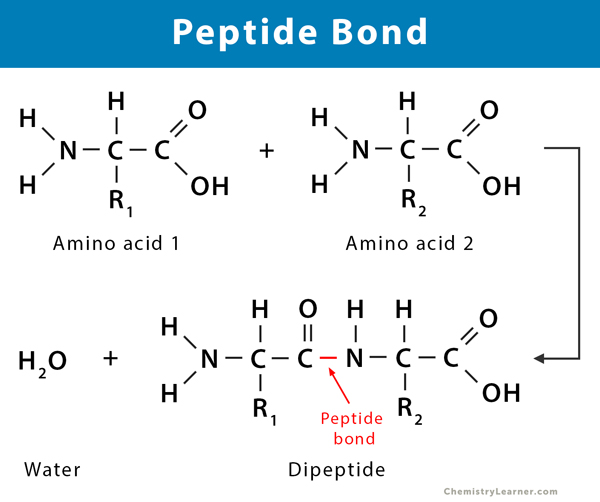
How are amino acids linked?
-OH from carboxyl group of one amino acid and hydrogen from amine group of a different amino acid undergo a condensation reaction where a peptide bond (-CONH) is formed
Gives rise to 'polarity' of proteins (due to N-H and carbonyl group (C=O)
This forms a polypeptide chain as more amino acids are linked
N terminus - amine group end
C terminus - carboxyl group end
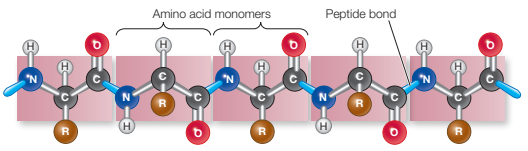
Describe primary structure of protein and the bonds that stabilise it.
amino acids joined to form polypeptide chain
peptide bonds
Describe secondary structure of protein and the bonds that stabilise it.
Polypeptide chains may form α helices or β pleated sheets.
hydrogen bonds
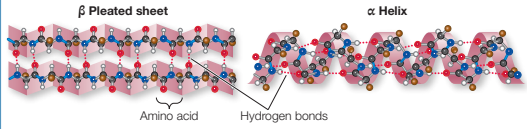
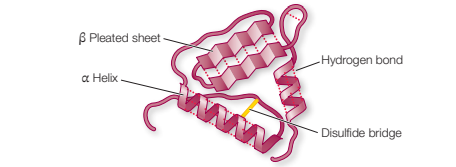
Describe tertiary structure of protein and the bonds that stabilise it.
Polypeptides fold and form shapes (3D structure), sometimes contain random coils
Hydrogen bonds, disulphide bridges and hydrophobic interactions
Shape determined primarily by sequence of amino acids
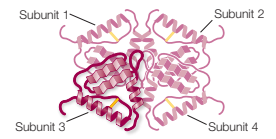
Describe quaternary structure of protein and the bonds that stabilise it.
Two or more polypeptides assemble to form larger protein
Hydrogen bonds; disulphide bridges; hydrophobic interactions; ionic bonds
Explain how each of the following could potentially cause denaturation of a protein:
pH change
temperature increase
addition of high concentration of a polar molecule
addition of a high concentration of a non-polar molecule
A pH change can cause ionization of exposed carboxyl and amino groups in the R groups of amino acids. This leads to differences in polarity which can alter the tertiary structure of the protein.
Temp increase can cause rapid molecular movements and thus can break hydrogen bonds and hydrophobic interactions
Polar molecules can disrupt hydrogen bonding
Non polar molecules may also disrupt hydrophobic interactions and alter structure.
How are disulphide bridges formed?
Two cystine molecules in a polypeptide chain form a disulphide bridge (-S-S-) by oxidation, covalent bond formed and H atoms removed

List the physical and chemical properties of lipids.
Triglyceride composed of glycerol and 3 fatty acids
hydrocarbons that are insoluble in water due to their many non-polar covalent bonds
Dissolve readily in organic solvents (e.g., hexane, benzene)
Compose mainly of C, H, O - smaller proportion of oxygen than carbs
May contain other elements (phosphorus, nitrogen)
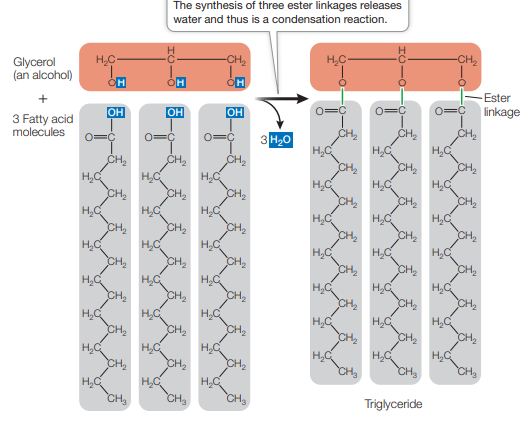
How are triglycerides made?
three condensation reactions
carboxyl group of a fatty acid bonds with a hydroxyl group of glycerol, resulting an ester linkage and the release of water
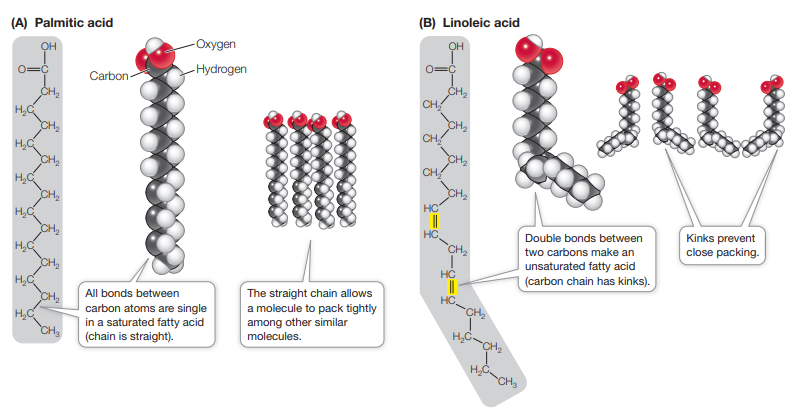
What is the main structural difference between saturated and unsaturated fatty acids?
Saturated
All bonds between C atoms are single
fatty acid molecules are relatively straight, and they pack together tightly
Generally make fats, solids, e.g., palmitic acid
Unsaturated
contain one or more double bonds (are inflexible)
Has kinks caused by double bonds which prevent close packing
Tend to be oils at room temp
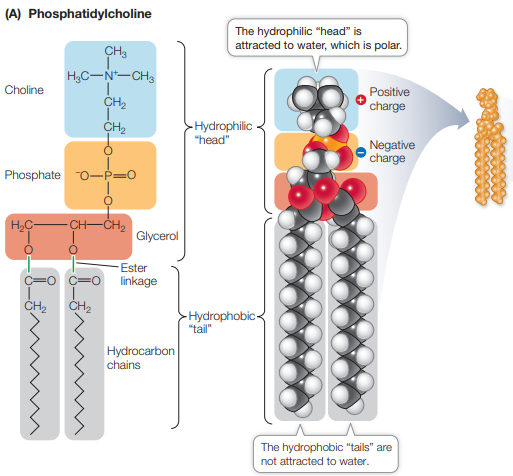
What is the structure of a phospholipid?
Hydrophilic head, hydrophobic tail
Amphipathic compound - part hydrophobic, part hydrophilic
Form closed vessels in aqueous environments