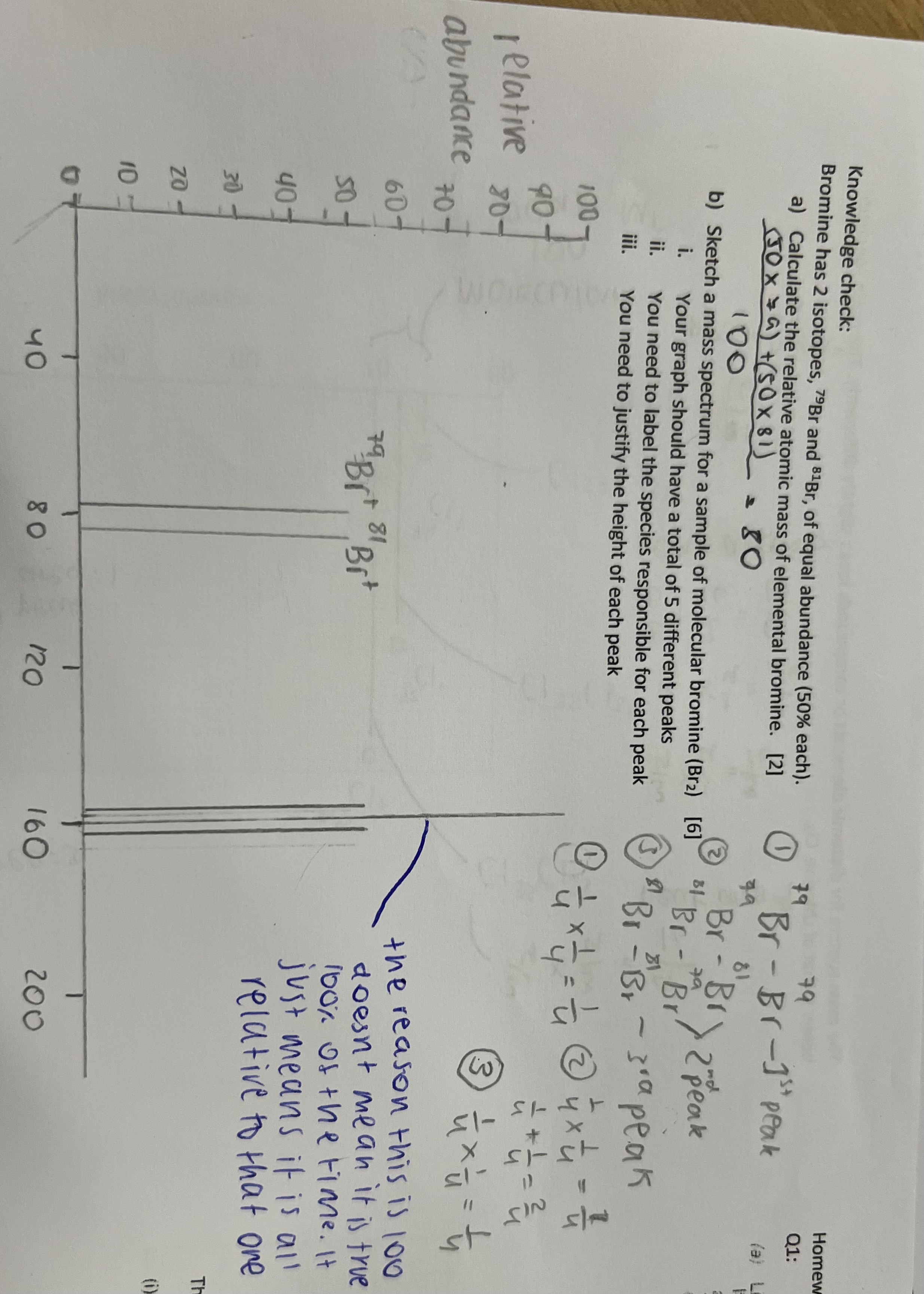
1.3 Chemical calculations
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
8 Terms
What is the definition of relative atomic mass (Ar)
The average mass of an element (taking into account all possible isotopes) relative to 1/12 the mass of one atom of carbon-12
What is the definition of relative isotopic mass
The mass of an individual isotope of an atom relative to 1/12 the mass of an atom of carbon-12
What is the definition of relative formula mass
The average mass of a molecule relative to 1/12 the mass of an atom of carbon-12 (sum of all the relative atomic mass in a formula)
How to calculate relative atomic mass using isotopes
(Mass of isotope 1 x abundance) + (mass of isotope 2 x abundance) /100
What is mass spectrometry used to find
-abundance of different isotopes for an element
-relative isotopic mass of a particular element
-relative atomic mass for an element overall
What happens in mass spectrometry
1.Ionisation - the atom or molecule is ionised to give a positive ion using an electron gun which bombards the sample with high energy electrons to knock an electron off the sample. A vacuum is used so the ions don’t hit any molecules in the air
2.Acceleration - the ions are accelerated through an electric field so that they all have the same kinetic energy
3.Deflection - the ions are deflected by a magnetic field according to their masses. The lighter they are the more they are deflected. The more the ion is charged the more it gets deflected
4.Detection - the beam of ions passing through the machine is detected electrically and amplified