redox. reactions
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
Chlorine + —— = sodium —— + ——- chlorate + water
Chlorine + cold dilute sodium hydroxide = sodium chloride +sodium chlorate + water
What are all the format of halate ions
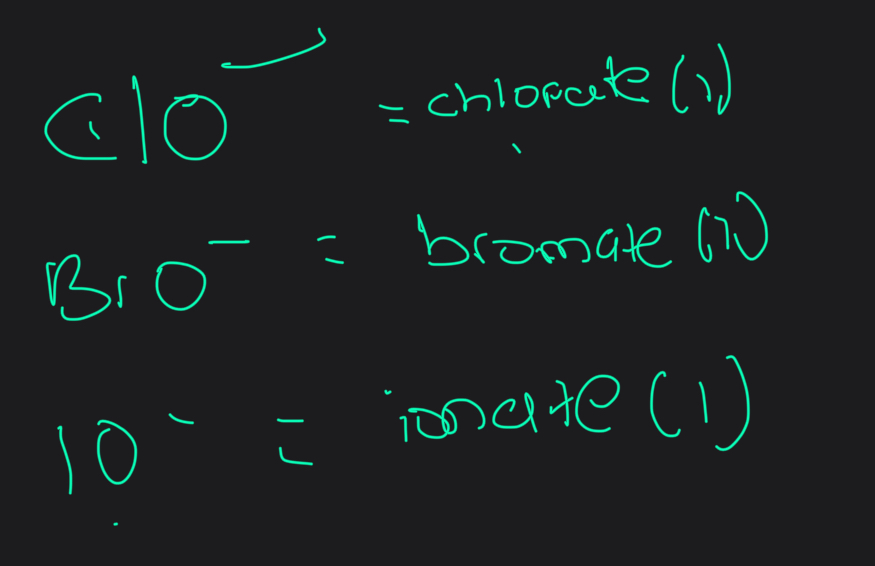
What is the oxidation number of halogen atoms in halate salts
+1
What is the ionic equation for chlorine+sodium hydroxide
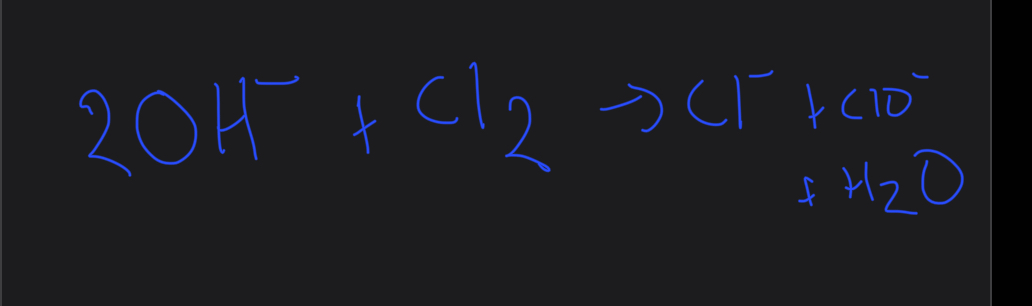
What are the observations for the reaction of chlorine and sodium hydroxide and how is it a redox reaction
Yellow green gas forms a colourless solution
Redox reaction
chlorine oxidised from cl2 to NaClO
Reduced cl2 - NaCl
What is a solution containing sodium chlorate used as
BLEACH
Sodium chlorate kills bacteria (Cl-)
Chlorate ions = bleaching (CLO-)
What is the equation for chlorine + water

What is hypochlorus acid used for? How do you relate this to the reversible reaction
Chloric acid is responsible for strong germicidal and bleaching power of wet chlorine
As HCLO kills microorganisms the p.o.e moves from left to right which makes there to be very little chlorine remains once HCLO has done its job
What is the reaction of chlorine and water in bright sunlight
UV breaks HCLO into HCL and o2
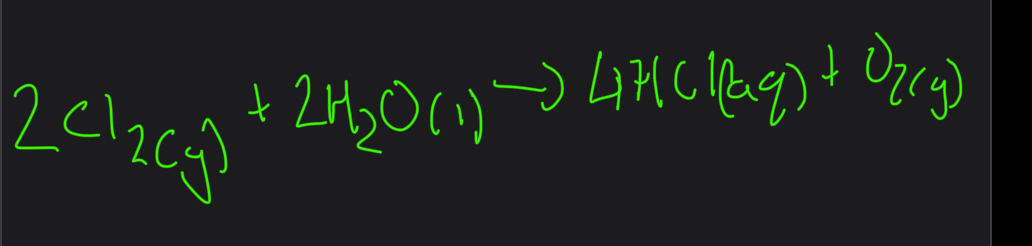
How does society assess whether the chemicals should be added to water supplies
They asses te advantages and disadvantages
Benefits of using chlorine in water treatment outweigh its toxic effects
What may be added to drinking water
Sodium fluroride
Helps strengthen enamel which helps prevent tooth decay but some object and say there could be risks
Chlorine sterilises water in pools and drinking water