Biology Exam 1 - Chapters 1-6
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
What is Science?
Science is a way of understanding the world, not a collection of facts. Science does not prove anything.
What is a Theory?
A theory is a set of statements or principles devised to explain a group of facts or phenomena. Theories are testable and are supported by a great body of evidence.
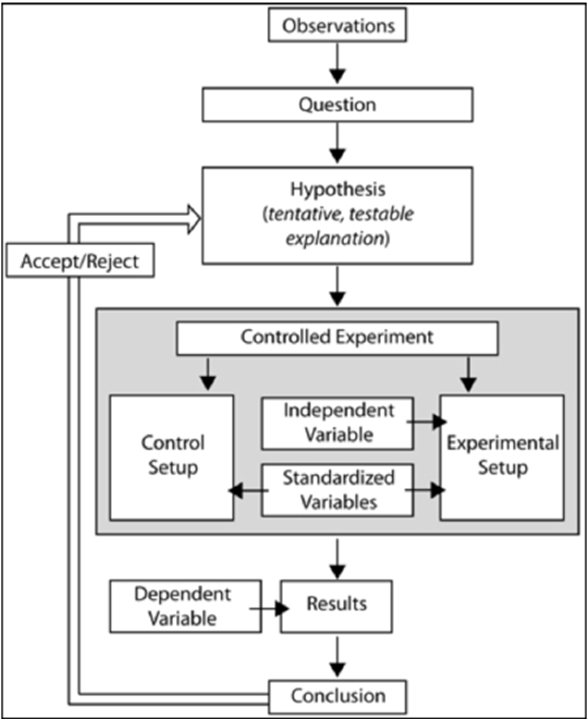
Scientific Method
Structure of an Atom
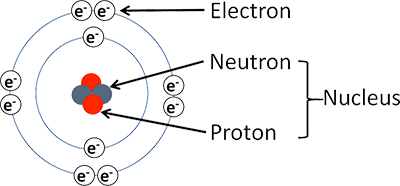
Electrons (neg. charge) orbit the nucleus, which is made up of protons (pos. charge) and neutrons (no charge). They don’t orbit randomly, but at a certain level from the nucleus called an electron shell.
What makes each element unique?
Every different atom has a characteristic number if protons in the nucleus, atoms with the same atomic number have the same chemical properties and belong to the same element. The mass number is the number of protons + neutrons of the most common isotope.
What are Isotopes?
Isotopes are two atoms of an element that differ in number of neutrons. Radioactive isotopes decay spontaneously, giving off particles and energy.
Energy levels of Electrons
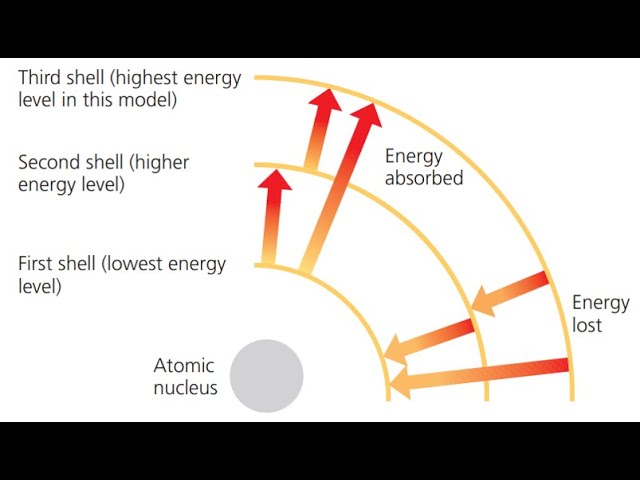
Electron Shells
Layered energy levels that surround a atoms nucleus where electrons are likely to be found, each shell can hold a specific maximum number of electrons.
Electron Distribution
The specific arrangement of electrons in shells or orbitals around an atoms nucleus
The first shell holds up to 2 electrons while the 2nd and 3rd hold up to 8
Covalent Bonds
The sharing of a pair of valence electrons by two atoms, the shared electrons count as part of each atom’s valence shell, two or more atoms held together by valence bonds constitute a molecule
Ionic Bond
A attraction between an anion (neg. charge) and a cation (pos. charge)
Hydrogen Bonds
A weak attraction between a slightly positive hydrogen atom in one molecule and a slightly negative atom in another molecule or the same molecule, causing molecules to stick together and giving water unique properties
Van der Waal Interactions
Weak, short-range electrostatic attractions between uncharged molecules or atoms, caused by temporary shifts in electron density that create brief positive and negative charges
Electronegativty
Atoms in a molecule attract electrons to varying degrees, Electronegativity is an atom’s attraction for the electrons in a covalent bond
The more electronegative an atom, the more strongly it pulls shared electrons toward itself
Polarity
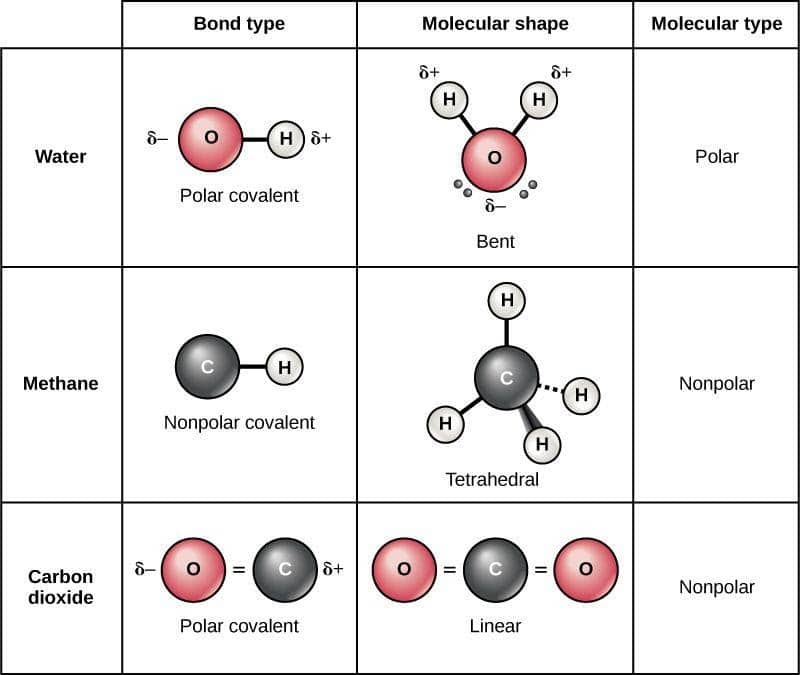
(a) Nonpolar covalent bond in hydrogen molecule
Electrons are halfway between the two atoms, shared equally
(b) Polar covalent bonds in water molecule
Electrons are not shared equally, so partial charges exist on the Oxygen and Hydrogen atoms
Properties of Water
Cohesive
Binding between like molecules, can bind to itself, results in high surface tension
Adhesive
Binding between unlike molecules, binding water to the side of a glass beaker
Denser as a liquid than a solid
Able to absorb large amounts of energy
water heats and cools very slowly compared to other compounds, this moderates the earths temperatures allowing for life
High Specific Heat
The amount of energy required to raise the temperature of 1 g of a substance by 1 degree C
Hydrophillic Substances
Ions and polar molecules that stay in solution (can dissolves in water), they stay can interact with water’s partial changes
Hydrophobic Substances
Are unchanged and nonpolar compounds, they do not dissolve in water
Acids
When water is a liquid, some of its molecules spontaneously separate into hydrogen ions and hydroxide ions, these ions can combine again to form water
Has a pH of less than &7
Bases
A substance with a pH higher than 7 that neutralizes acids and accepts hydrogen ions
The pH Scale
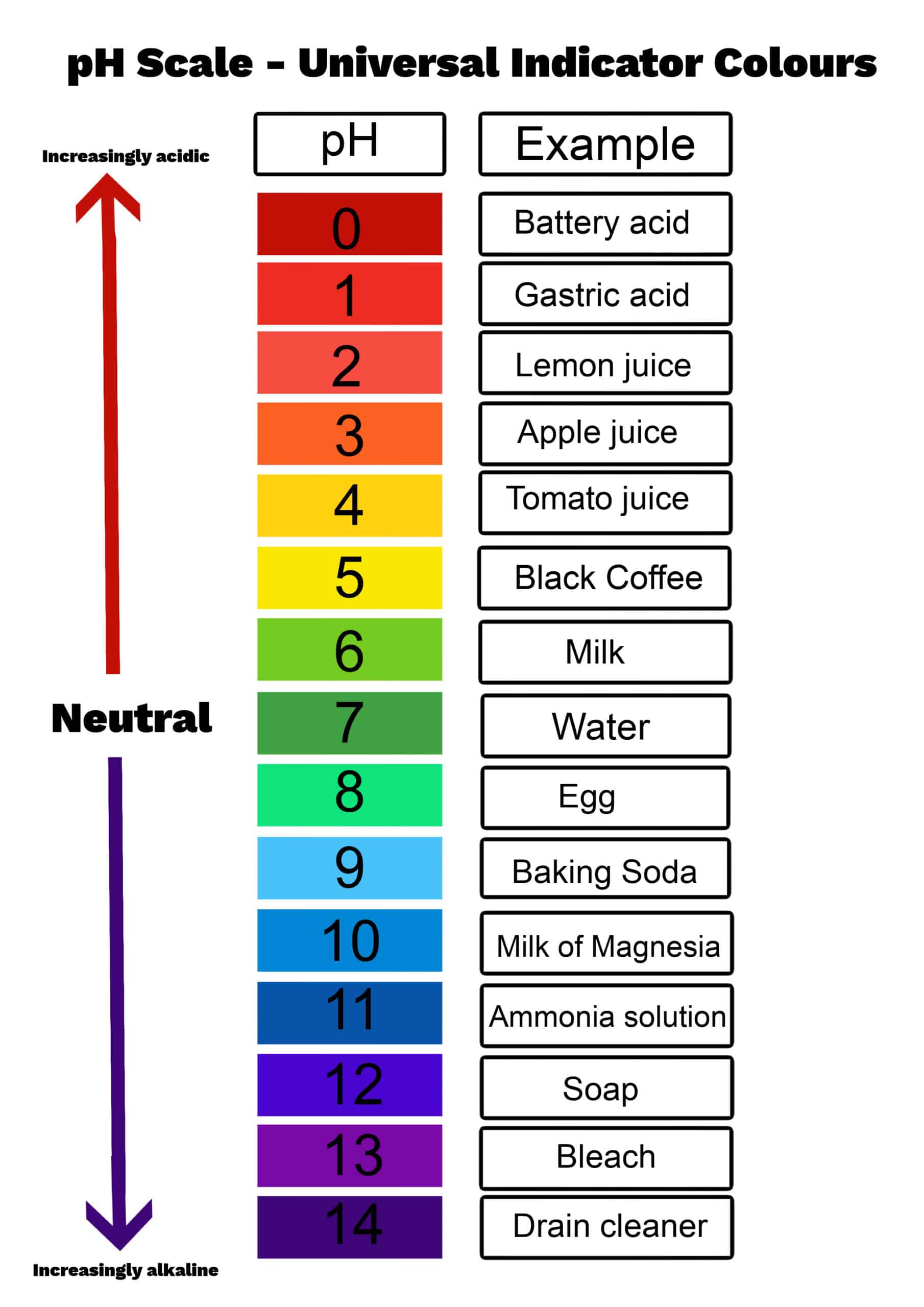
The pH scale is logarithmic:
pH = -log [H^+]
Greater H^+ concentration = lower pH = more acidic
Lower H^+ concentration = higher pH = more basic
Buffers
The internal pH of most living cells must remain close to pH 7, Buffers are substances that minimize changes in concentrations of H^+ and OH^- in a solution
Most buffers consist of an acid-base pair that conjugate base combines with H^+
Chemical Properties of Carbon
Carbon is the most versatile atom, it has four valence electrons and can form four covalent bonds
Carbon-containing molecules can form an almost limitless array of molecular shapes with different combinations of single and double bonds
Identification of Functional Groups
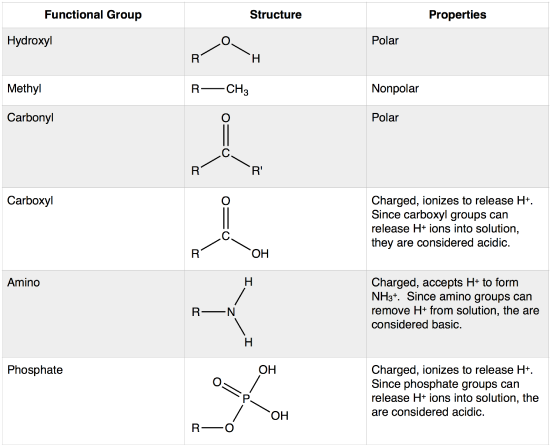
Components of organic molecules that are most commonly involved in chemical reactions, the number and arrangement of functional groups give each molecule its functional groups
Enzymes
A protein that acts as a biological catalyst, speeding up specific chemical reactions in living cells without being used up, they work by binding to reactant molecules at a special site, making it easier for bonds to break and form
What is the Active Site of Enzymes?
A specific, small, 3D region (cleft or groove) on the protein's surface where substrate molecules bind and undergo a chemical reaction
DNA vs RNA
DNA is a double-stranded helix while RNA is a single-stranded
DNA has deoxyribose sugar while RNA has ribose (which has an extra oxygen atom)
DNA acts as long-term storage for genetic info. while RNA copies and transfers the genetic info
DNA is located within the nucleus, RNA is produced in the nucleus but operates in the cytoplasm
What are the differences between Starch, Glycogen, and Cellulose?
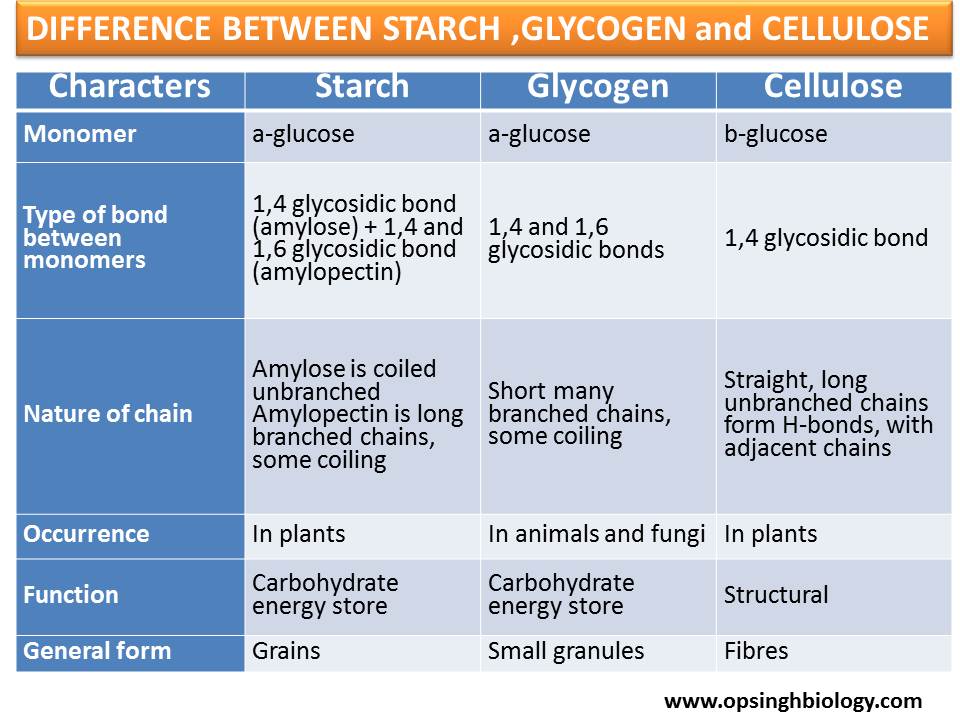
Starch stores energy in plants, glycogen stores energy in animals, and cellulose provides support in plant cell walls
Starch and glycogen are 𝛼-glucose polymers, while cellulose is a 𝛽-glucose polymer
How does lipid structure affects membrane permeability?
It controls membrane permeability by acting as a hydrophobic barrier that favors small, nonpolar molecules while blocking ions and large, polar molecules. Tight packing of saturated fatty acids decreases permeability, whereas kinked, unsaturated tails increase fluidity and permeability.
Saturated vs. Unsaturated H-C tails
Saturated hydrocarbon (H-C) tails contain only single bonds, maximizing hydrogen atoms, resulting in straight chains that pack tightly, forming solids.
Unsaturated tails have one or more double bonds (𝐶=𝐶), creating "kinks" that prevent tight packing, leading to liquid fats/oils
Selective Permeability of Lipid Bilayers
It allows only substances meeting certain criteria to pass through it unaided. In the case of the cell membrane, only relatively small, nonpolar materials can move through the lipid bilayer
Definition of the fluid-mosaic model
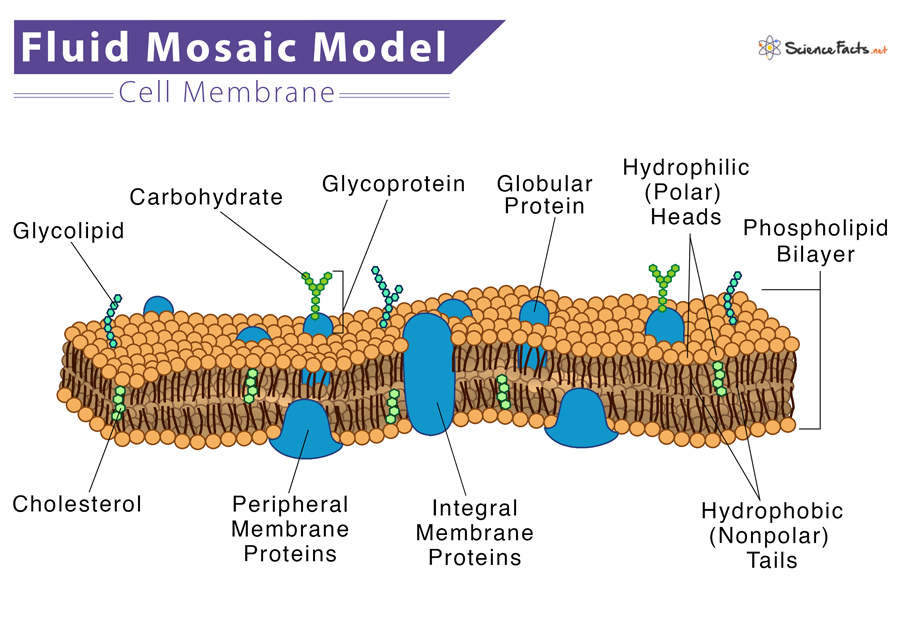
Some proteins are inserted into the lipid bilayer thus making the membrane a fluid, dynamic mosaic of phospholipids and proteins
Diffusion
The overall movement of particles from areas of high concentration to areas of low concentration. In living things, diffusion allows substances to move in and out of cells
Osmosis
The natural, passive movement of water molecules through a semipermeable membrane from a region of lower solute concentration (more water) to a region of higher solute concentration (less water). It acts to equalize the concentration of solutions on both sides of the membrane.
Channel Membrane Proteins
It is specialized proteins spanning the cell membrane that act as selective, tunnel-like pores.
They allow specific small molecules and ions to passively diffuse across the membrane down their concentration gradient without using energy.
They are crucial for transporting water, ions, and maintaining cell homeostasis.
Gated Membrane Protein
It is a specialized channel or carrier protein in a cell membrane that acts as a "door," controlling the passage of specific ions or molecules in and out of the cell.
It remains closed, opening only in response to specific stimuli—such as electrical signals, chemical binding, or mechanical pressure.
Carrier Membrane Proteins
It is a specialized proteins spanning the cell membrane that transport specific molecules (like sugars or amino acids) by binding to them, changing shape, and releasing them on the other side.
They facilitate both passive transport (down concentration gradients) and active transport (against gradients).
Pumps Membrane Proteins
It is a specialized, energy-consuming proteins embedded in the cell membrane that move ions or molecules against a concentration gradient (from low to high concentration).
They function like active transport mechanisms, often using ATP to power the movement of substances across the membrane.
What is an electrochemical gradient?
It is the difference between the membrane potential (V m) and the equilibrium potential of an ion (E ion), which determines the passive diffusion of that ion through an open channel. It serves as the driving force for ion movement across the membrane.
Which membrane proteins build these electrochemical gradients?
Electrochemical gradients are primarily built by transmembrane proteins known as ion pumps or active transporters, which use energy (often ATP) to move ions against their concentration gradients. Key proteins include the sodium-potassium pump, hydrogen pumps (proton pumps), and calcium pumps.
Know the step-by-step process of the sodium-potassium pump
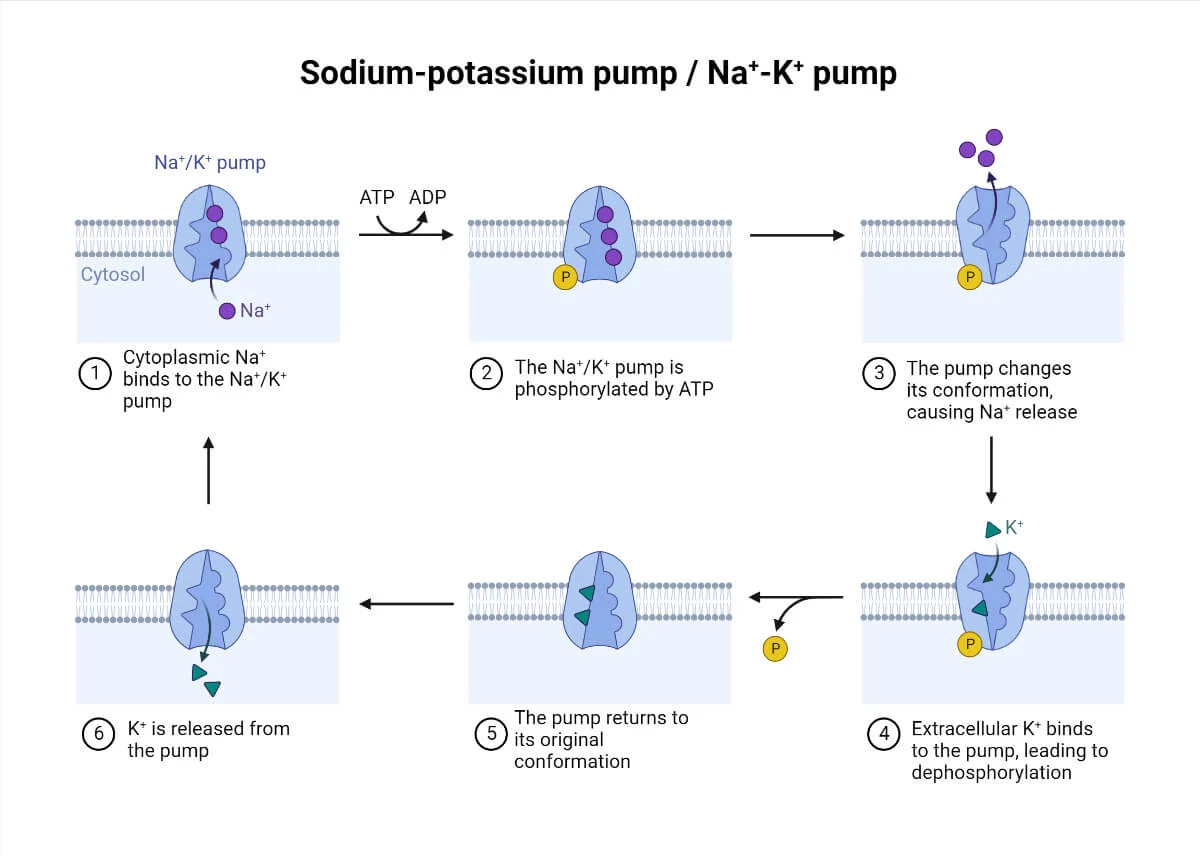
binding 3 sodium ions internally
breaking down ATP to change shape
releasing sodium outside
binding 2 potassium ions
returning to the original shape to release potassium inside.