Auer and Stick Chapter 1: Shock: Pathophysiology, Diagnosis, Treatment, and Response to Trauma
1/97
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
98 Terms
Definition of Shock
Progression of a cascade of events that begins when cells or tissues are deprived of an adequate energy source because of oxygen deprivation
Occurs as a result of inadequate tissue perfusion
Lack of an adequate energy supply leads to the buildup of waste products and failure of energy-dependent functions, release of cellular enzymes, and accumulation of calcium and reactive oxygen species (ROS) resulting in cellular injury, and ultimately cellular death
Activation of the inflammatory, coagulation, and complement cascades results in further cellular injury and microvascular thrombosis
The amplification of these processes coupled with increased absorption of endotoxin and bacteria (as a result of liver and gastrointestinal dysfunction) leads to the systemic inflammatory response syndrome (SIRS), multiple organ dysfunction (MOD), and if uncontrolled, ultimately death
Major factors affecting blood flow
Circulating volume
Cardiac pump function
Vasomotor tone or peripheral vascular resistance
What determines the blood flow to tissues and what is it regulated by?
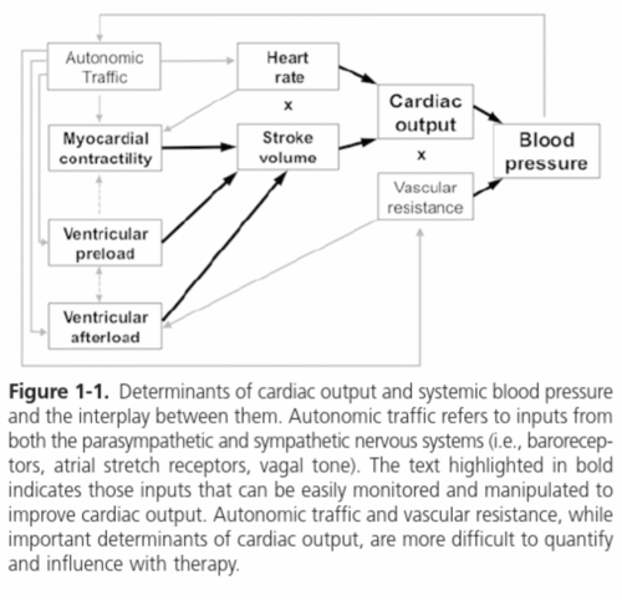
Cardiac output (CO)
Regulated by the stroke volume (SV)
SV is the result of:
Ventricular preload (amount of blood returning from the body and entering the heart)
The myocardial contractility (systolic cardiac function)
Ventricular afterload (the force the heart must overcome to push blood across the aortic and pulmonic valves into the peripheral or pulmonary vasculature)
What is ventricular preload directly affected by?
Circulating blood volume or amount of blood returning to the heart
Causes of decreased preload
Hypovolemia
Decreased ventricular filling time (resulting from tachycardia)
Impaired ventricular relaxation
Decreases in vasomotor tone and vasodilation which results in pooling of blood in capacitance vessels and decreased return to the heart
- Total volume of blood remains unchanged but the effective circulating volume decreases
What determines myocardial contractility?
The rate of cross-bridge cycling between actin and myosin filaments within cardiomyocytes
What affects ventricular afterload?
Directly affected by vasomotor tone or peripheral vascular resistance
If vascular resistance or tone increases (hypertension), afterload also rises with a resultant fall in CO and tissue perfusion
Determinants of cardiac output and systemic blood pressure diagram
Types of Shock
Hypovolemic shock
Cardiogenic shock or pump failure
Distributive shock or microcirculatory failure
Obstructive shock
Hypovolemic shock
Result of a volume deficit
Due to blood loss, third-space sequestration, or severe dehydration
Untreated can result in microcirculatory failure as oxygen debt causes muscle dysfunction and relaxation
Can result in myocardial failure as perfusion deficits affect energy supply to the myocardium resulting in decreased myocardial contractillity
Cardiogenic shock or pump failure
The cardiac muscle cannot pump out adequate SV to maintain perfusion
Aggressive fluid therapy may worsen clinical signs
Distributive shock or microcirculatory failure
Vasomotor tone is lost
Results in a dramatic decrease in both blood pressure and venous return
Common causes include neurogenic shock, septic shock, and anaphylactic shock
Fluid therapy is indicated to help restore perfusion
Obstructive shock
Obstruction of ventilation or of CO
Most commonly caused by tension pneumothorax, pericardial tamponade, diaphragmatic hernia, or severe abdominal distension causing vena cava obstruction leading to inadequate ventricular filling, decreased preload, and consequently, decreased SV and CO
Stages/Severities of Shock
Compensated Shock
Decompensated Shock
What is compensated shock?
Early or mild shock, during which the body's response mechanisms are able to restore homeostasis
Pathophysiology of Compensated Shock
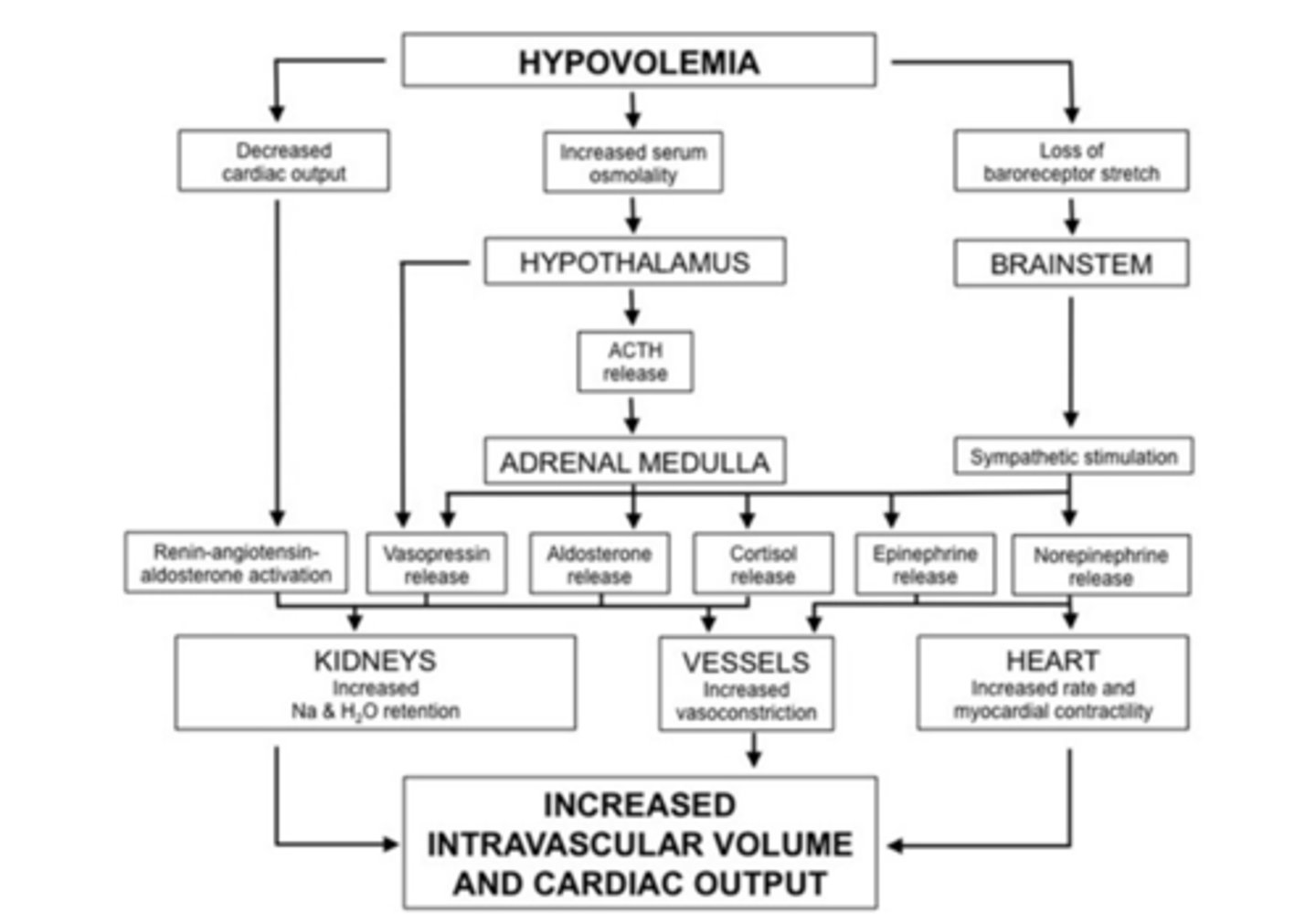
As blood volume is depleted, pressure within the vessels falls
Baroreceptors and stretch receptors located in the carotid sinus, right atrium, and aortic arch sense this fall in pressure. These receptor responses act to decrease inhibition of sympathetic tone while increasing inhibition of vagal activity and decreasing the release of atrial natriuretic peptide (ANP) by cardiac myocytes
The increase in sympathetic tone and fall in ANP results in vasoconstriction which increases total peripheral resistance and thereby increases blood pressure
Increased sympathetic activity at the heart increases heart rate and systolic cardiac function, hence increasing SV and CO
Peripheral chemoreceptors stimulated by local hypoxemia respond by enhancing this vasoconstrictive response
Cerebral and cardiac flow is preferentially maintained in mild to moderate hypovolemia
A decrease in renal perfusion results in secretion of renin from juxtaglomerular cells located in the wall of the afferent arteriole
Renin stimulates the production of angiotensin I, which after conversion to angiotensin II, increases sympathetic tone on peripheral vasculature and promotes aldosterone release from the adrenal cortex
Aldosterone restores circulating volume by increasing renal tubular sodium and water reabsorption
Arginine vasopressin (AVP, previously known as antidiuretic hormone, ADH), released from the posterior pituitary gland in response to decreased plasma volume and increased plasma osmolality, is a potent vasoconstrictor and stimulate increased water reabsorption in the renal collecting ducts
An increase in thirst and a craving for salt is mediated by both the renin-angiotensin-aldosterone system (RAAS) and a fall in ANP
Autonomic traffic
The interplay between the parasympathetic and sympathetic nervous system
Hyperdynamic stage of shock
Tachycardia
Increased SV (increased pulse pressure)
shortened CRT due to compensatory responses
Where do the greatest vasoconstrictive responses occur during compensated shock?
Viscera
Integument
Kidney
Physiologic compensatory responses to hypovolemia chart
What is decompensated shock?
With more severe blood loss, compensatory mechanisms become insufficient to maintain arterial blood pressure and perfusion of vital organs
Decompensated Shock Pathophysiology
Ischemia to the more vital organs including the brain and myocardium develops
Blood pressure may be maintained, but clinical signs including resting tachycardia, tachypnea, poor peripheral pulses, and cool extremities are present
Mild anxiety and sweating may be present from increased sympathetic activity
Urine output and central venous filling pressure will drop
Severe vasoconstriction further worsens the ischemia such that the energy supplies are inadequate and cellular functions begin to fail
Accumulation of waste products of metabolism (lactate and carbon dioxide) cause progressive acidosis and further cellular dysfunction
Loss of critical energy-dependent functions, including enzymatic activities, membrane pumps, and mitochondrial activity, leading to cell swelling and releases of intracellular calcium stores
Cytotoxic lipids, enzymes, and ROS released from damaged cells further damage cells, triggering inflammation
Inflammatory cell and platelet influx into the tissue, the formation of neutrophil extracellular traps (NETS), and activation of the arachidonic acid cascade and the complement cascade, cause further cellular injury
Mitochondrial failure, calcium releases, and reperfusion, if present, further increase production (and decrease scavenging) of ROS
Endothelial cell damage, including loss of the endothelial glycocalyx layer, results in local tissue edema as the result of protein and fluid leakage
Exposure of subendothelial tissue factor further activates the coagulation and complement cascades
Formation of microthrombi coupled with coagulopathy impedes blood flow to the local tissues
Lack of energy supplies, + accumulation of toxic metabolites + microthrombi formation + inflammatory injury -> smooth muscle failure and vasodilation
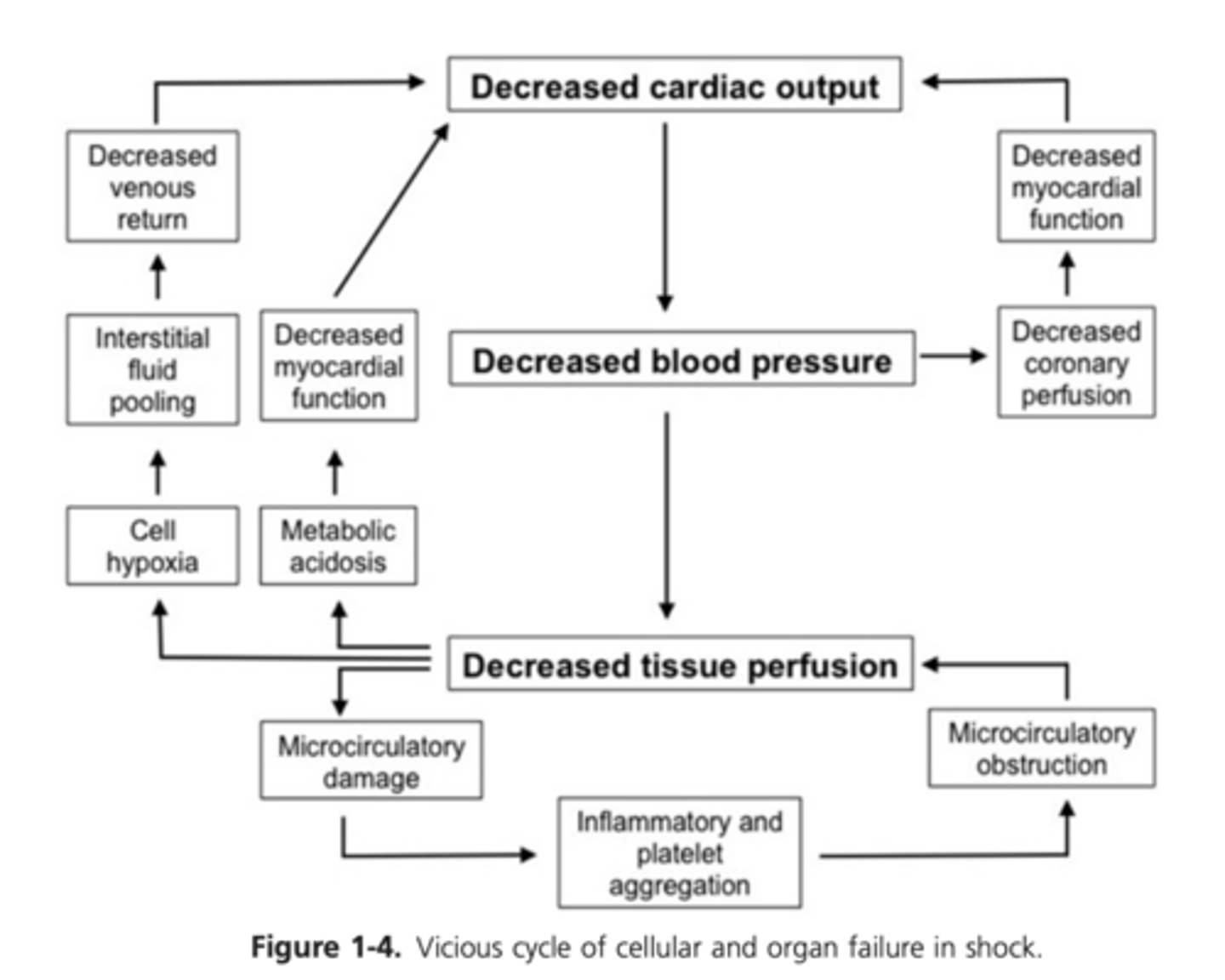
End results - pooling of blood in peripheral tissue beds and additional decreases in blood pressure, venous return, CO, and perfusion , ultimately resulting in organ failure
Failure of GIT tract - loss of mucosal barrier integrity -> protein and fluid loss, endotoxin absorption, bacterial translocation
Renal ischemia -> renal tubular necrosis, the inability to reabsorb solutes and water, inability to excrete waste products
Continued drop in blood pressure and venous return decreases coronary blood flow
Cardiac muscle ischemia leads to decreased cardiomyocyte contractility and CO and ultimately to further deterioration of coronary artery blood flow
Acidosis and ischemia accentuate the depression of cardiac muscle function
If blood flow is restored, reperfusion injury results from the activated cellular and immunochemical products washed into the venous circulation and leads to SIRS, MOD, and death
Diagram - Cellular Cascade of Events that Occur as the Result of Hypovolemia, Poor Perfusion, and Decreased Oxygen Delivery
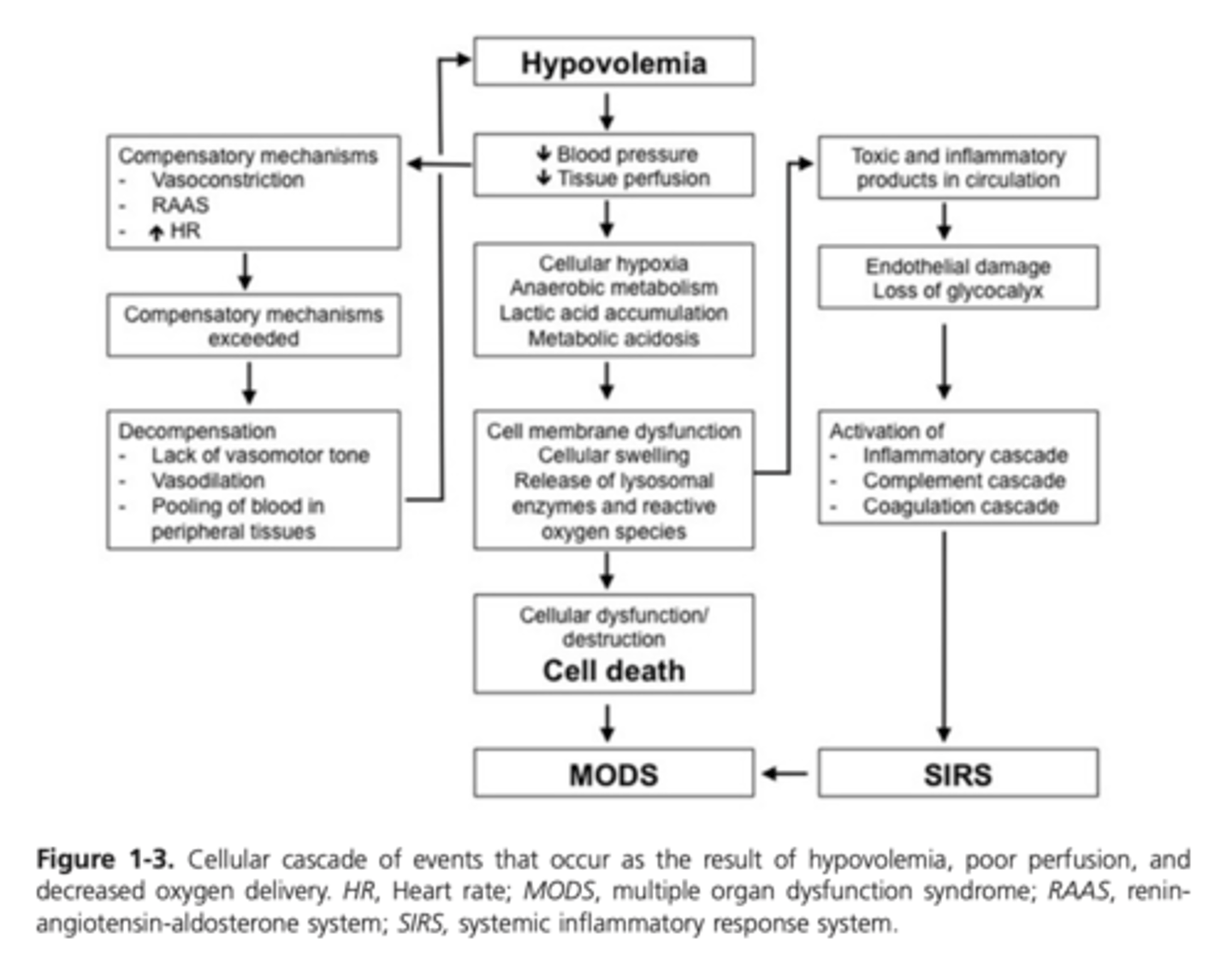
Diagram: Cycle of Cellular and Organ Failure in Shock
How many classes is shock divided into? What do these classes depend on?
4 classes
Depends on volume of blood loss
Class I Shock
Mild blood loss of less than 15% total blood volume
The body is capable of restoring volume deficits via compensatory responses and there may be little to no change in the physical findings other than a drop in urine output
Blood pressure is maintained
Class II Shock
15-30% blood loss
Clinical signs become apparent
Onset of hyperdynamic shock
Tachycardia, tachypnea, and a bounding pulse (increased CO and peripheral vascular resistance)
Mental agitation or anxiety
Increased sympathetic output results in sweating and pupil dilation
Compensatory mechanisms can normalize blood pressure, but perfusion deficits will persist and can be detected on a blood gas (increased lactate and a high anion gap metabolic acidosis)
Class III Shock
If blood loss continues or hypovolemia persists, compensatory mechanisms can be insufficient to restore circulating volume and hypodynamic/decompensatory shock beings
Moderate hypovolemic shock
Profound tachycardia and tachypnea, anxiety, agitation
Urine output may cease, jugular filling and CRT are prolonged, pulse pressure is weak, and extremity temperatures are decreased
Lactic acidosis on blood gas
Blood pressure drops despite increases in heart rate, cardiac contractility, and total peripheral resistance
Continued cellular hypoxia and acidosis result in failure of compensatory mechanisms causing peripheral vasodilation and decreased myocardial contractility
Decreased coronary artery perfusion causing decreased cardiac function, resulting in decreased CO and a further drop in perfusion
Class IV Shock
If uncontrolled, progresses from tachycardia and anxiety to bradycardia, obtundation, anuria, profound hypotension, circulatory collapse, and death
Uncompensated life-threatening hemorrhagic shock
Delivery of oxygen formula
DO2 - delivery of oxygen
CaO2 - content of oxygen in the arterial blood
CO - amount of blood perfusing the tissue
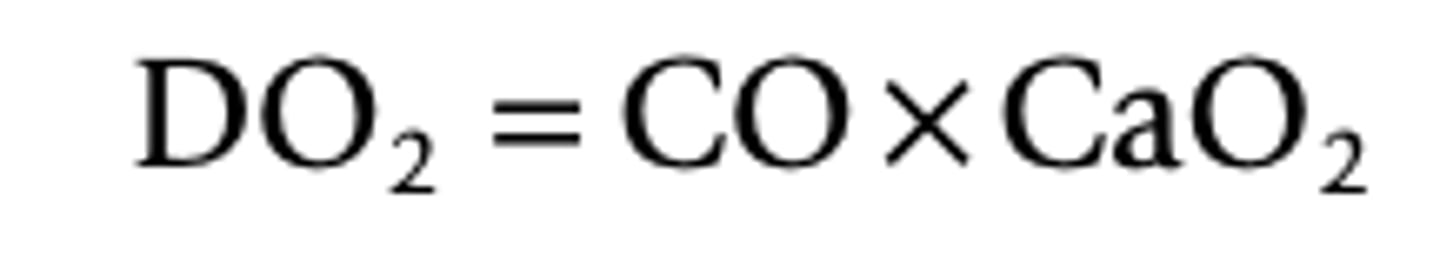
What determines the content of oxygen per volume of blood?
The amount of hemoglobin (Hb) or red cell mass and the saturation of that Hb (SaO2)
What percentage of the volume of crystalloids will rapidly diffuse out of the vascular space into the interstitial and intracellular space?
Approximately 80%
What must the replacement volumes be when using crystalloids?
4-5x greater than the volume loss
What will crystalloid replacement result in in acute blood loss or hypovolemic states?
Excess total body water and extreme excesses of sodium and chloride
What happens if the electrolyte concentration of isotonic crystalloids differ from those in the intracellular space?
Cellular swelling will ensue which affects the activity of various protein kinases, increases intracellular calcium concentrations, alters ion pump activity, membrane potential, and cytoskeletal structure, and activates phospholipase A2
What can high volumes of crystalloids cause?
Can trigger or potentiate an inflammatory response and have a negative impact in the face of ischemia and reperfusion
Large-volume infusions can result in abdominal compartment syndrome, acute respiratory distress syndrome, congestive heart failure, gastrointestinal motility disturbances, and dilutional coagulopathy
What is the current recommended crystalloid bolus volume recommendations by the Advanced Trauma Life Support and current Surviving Sepsis Campaign guidelines?
Lower-volume bolus crystalloid therapy
30 mL/kg within the first 3 hours after presenation
What is the current recommended fluid therapy approach for hypovolemic shock?
Isotonic crystalloid administration
Initially a rapid 20 mL/kg (10 L for a 500 kg horse) bolus is administered over the first 30-60 minutes with assessment of the cardiovascular system at regular intervals to monitor the response
Types of Fluids
Isotonic Crystalloids
Hypertonic Crystalloids
Colloids
Whole Blood
Vasopressors
Isotonic Crystalloids
Designed to be replacement fluids, not maintenance fluids
In cases of moderate to severe blood loss, infusion of large volumes of crystalloids alone can cause dilutional anemia and hypoproteinemia but the oxygen-carrying capacity (red blood cell mass) will remain unchanged or become improved Intravenous crystalloid fluid therapy should never be withheld, even when the PCV/TS are reduced
Hypertonic Crystalloids
7.2% hypertonic saline solution most common formulation At this concentration, HSS has approximately 8x the tonicity of plasma Will expand the intravascular space by approximately twice the amount infused pulling fluid from the intracellular and interstitial spaces
Majority of fluid and electrolytes will ultimately diffuse into the interstitial space
HSS blunts neutrophil activation and may alter the balance between inflammatory and antiinflammatory cytokine responses to hemorrhage and ischemia
Recommended dose is 2-4 mL/kg or 1-2 L for a 500 kg horse
Where does hypertonic saline solution principally pull volume from?
The intracellular space, not the interstitial space
Particularly beneficial in shock state where endothelial cell volume rises with loss of membrane pump function Decrease in endothelial cell volume increases capillary diameter and improves perfusion
What is the colloid oncotic pressure of normal equine plasma?
About 20 mmHg
What do colloids with a high colloid oncotic pressure (COP) do?
Draw additional fluid into the intravascular space
How much does hydroxyethyl starch (HES) increase intravascular volume?
COP ~30 mmHg
Increases intravascular volume by an amount that is greater than the infused volume
Natural colloids
Plasma
Whole blood
Bovine albumin
What do natural colloids provide?
Protein such as albumin, antibodies, critical clotting factors, antithrombin 3, and other plasma constitutents
How often do hypersensitivity reactions occurs in horses receiving plasma?
Occur in up to 10% of horses receiving plasma
Synthetic Colloids
HES (hetastarch and tetrastarch)
Dextrans
How does elimination of hydroxyethyl starch occur?
Renal excretion and extravasation
Larger molecules are degraded over time by a-amylase
How are different HES products differentiated?
By the molecular weight (high, medium, low)
By the molar substitution ratio (number of hydroxyethyl groups per glucose molecule) of starch molecules
What type of HES is most commonly used now?
Low molecular weight and molar substitution HES (6% HES, 130 kDA/0.4 tetrastarch) has replaced the previous higher molecular weight and molar substitution HES (6% HES, 130 kDA/0.75 hetastarch) because of concerns in human medicine over higher mortality, increased risk of renal replacement therapy, and coagulopathies
How can you assess the response to HES?
You must measure COP because administration of HES results in a dilution of TS or TP
When is whole blood an ideal replacement fluid?
In patients with hypovolemic shock as a result of severe blood loss
What does whole blood provide?
Provides clotting factors and prevents dilutional coagulopathy
By providing RBCs and protein, it helps to retain fluids within the intravascular space and improve the oxygen carrying capacity of the blood
What is the estimated circulating blood volume of an adult horse?
7-9% of the total body weight or 35-45 L for a 500 kg horse
When do clinical signs of blood loss occur?
After the loss of 15% of circulating blood volume or approximately 6 L during an acute hemorrhage
What is the maintenance fluid requirement for an adult horse? How fast can it be replaced?
2-4 mL/kg/hr
Can be replaced over 24 hours
Should normalization of blood pressure be the goal in situations where bleeding is uncontrolled?
No
This may promote continued bleeding (permissive hypotension, i.e. mean arterial blood pressure >65 mmHg rather than >90 mmHg
Vasopressors
Dobutamine
Norepinephrine
Vasopressin
Dobutamine
Most commonly used drug in awake, adult horses
Strong B1-adrenoreceptor agonist with relatively weaker B2 and a-adrenoreceptor affinity
What is the main use of dobutamine?
Improve oxygen delivery to the tissues via its positive inotropic action
What are the recommended dosages of dobutamine?
1-5 ug/kg/min
Higher doses have been reported to cause hypertension, tachycardia, and arrhythmias in the adult horse
Norepinephrine
Useful in restoring adequate organ perfusion in vasodilatory shock in neonatal foals
Strong B1 and a-adrenergic affinity, resulting in vasoconstriction and increased cardiac contractility
Use in combination with dobutamine to improve arterial pressure and urine output in persistently hypotensive foals
Vasopressin
Released from the pituitary gland following periods of hypotension
Powerful vasoconstrictor in addition to its effects in the kidney
Occasionally used in horses under GA if hypotension does not respond to other vasopressors
Central Venous Pressure
Assesses cardiac function blood volume, and vascular resistance or tone
Jugular fill is a crude assessment of venous return or CVP Holding off the jugular vein should result in visible filling within 5 seconds in a normally hydrated horse that is standing with an elevated head
A more accurate estimate of CVP can be obtained with a water manometer, attached to a large-bore jugular catheter and placed at the level of the heart base or point of the shoulder
If clinical signs are deteriorating despite a normal CVP, hypovolemia alone is not the cause Low CVP can occur with hypovolemia or a drop in effective circulating volume, as occurs with distributive shock Cardiogenic shock, fluid overload, or pericardial effusion can result in an elevated CVP because forward failure of the cardiac pump results in backup of blood within the venous side of the system Jugular veins may be distended even with the head held high
What is normal CVP in standing horses measured by inserting a catheter into the cranial vena cava/right atrium?
7-12 mmHg
Is CVP measured in the jugular vein with a standard IVC accurate?
No, will result in falsely elevated CVP
When will CVP fall to zero
With a loss of 15-26% circulating volume when measured somewhere within the cranial vena cava/right atrium
Following loss of 4-6% of body weight
What is normal urine production?
Approximately 1 mL/kg/hr or more
What amount of urine production suggests a significant volume depletion?
Less than 0.5 mL/kg/hr
Fluid therapy is indicated to prevent renal ischemia
Arterial Blood Pressure
Reflection of CO and total vascular resistance
A normal blood pressure does not rule out hypovolemic shock, but a low blood pressure is often an indicator of significant blood loss
Treatment goals should be to maintain the MAP above 65 mm Hg to ensure adequate perfusion of the brain
Can be measured directly via arterial catheterization of the transverse facial artery in the adult horse or the transverse facial, metatarsal, radial, and auricular arteries in neonates
Indirect measurement of the blood pressure can be achieved using the coccygeal artery in adult horses and the metatarsal artery in foals
When does blood pressure fall below normal?
Because of the compensatory increase in peripheral resistance, blood pressure does not consistently fall below normal until the blood volume is profoundly decreased (30% or more)
What is a normal MAP in a healthy awake horse obtained using indirect measurement at the coccygeal artery?
105-135 mmHg
What is lactate a product of?
L-lactate is the end product of the anaerobic metabolism of glucose
How much ATP does aerobic metabolism of glucose result in?
36 moles of ATP per molecule of glucose
Type A Hyperlactatemia
Inadequate oxygen delivery to the tissue increase blood lactate concentrations
How much ATP does anaerobic metabolism of glucose result in
In the absence of adequate oxygen to meet energy demands, results in 2 moles of ATP
Type B hyperlactatemia
Can develop despite appropriate tissue oxygenation as a result of hepatic dysfunction (impaired clearance), pyruvate dehydrogenase inhibition, catecholamine surges, and sepsis or SIRS
Increase in lactate concentrations generally less than what is seen in horses with hypovolemia
What is the normal response to a decrease in perfusion or CO?
Increase the oxygen extraction ratio (O2ER) of the blood as it moves through the capillaries
Oxygen Extraction Formula
SaO2 - oxygen saturation of arterial blood
SvO2 - oxygen saturation of venous vlood
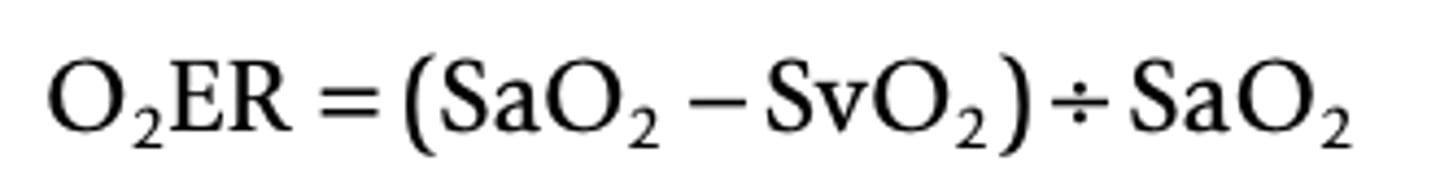
How can oxygen extraction be determined?
Can be determined by measuring central venous saturation and arterial oxygen saturation
O2ER can also be estimated by measuring jugular venous saturation and by using a pulse ox to assess arterial oxygen saturation
Oxygen Extraction in the Normovolemic, Healthy Individual
DO2, far exceeds oxygen need or uptake (VO2), and the O2ER ranges from 20% to 30% (one of the four O2 molecules from each Hb is removed)
Oxygen Extraction in Decreased Perfusion
The O2ER can increase with decreased perfusion to a maximum of 50-60% (two of the four O2 molecules are removed) at which point oxygen delivery becomes supply or flow dependent and a further drop in perfusion will result in a decrease in oxygen delivery
Mixed Venous Partial Pressure of Oxygen
PvO2 - mixed venous partial pressure of oxygen
In low-perfusion states, more oxygen is extracted per volume of blood and, consequently, PvO2 will fall
Mixed venous blood is ideally measured by catheterizing the pulmonary artery because a sample from the jugular vein or cranial vena cava only assesses venous blood returning from the head
Jugular venous pressure of oxygen (PjvO2) is usually greater than PvO2 in the shock state, but it is still useful in estimating global tissue hypoxemia
Increased venous partial pressure of oxygen in the presence of significant perfusion or supply deficits (DO2) can signify impaired oxygen consumption caused by mitochondrial or cellular dysfunction
What is normal jugular venous partial pressure of oxygen (PjvO2)?
40-50 mmHg
What is normal jugular venous saturation of oxygen (SjvO2)?
65-75%
Cardiac Output
Evaluates both volume return to the heart and cardiac function
Gold standard for CO monitoring is the pulmonary thermodilution method, which requires catheterization of the pulmonary artery
An alternative technique, lithium dilution - injection of lithium dye into the venous system results in generation of a lithium concentration-time curve which is used to calculate CO
Transcutaneous 2D echocardiography Volumetric methods (four chamber area length, Simpson, and bullet) for measuring CO have been shown to have better agreement with lithium dilution CO than Doppler-based methods
Greatest benefit in cases that fail to respond as expected to initial resuscitation efforts, cases with complex disease involving multiple organ systems, or those with cardiac disease
Regional Perfusion
Non-invasive measures of regional tissue perfusion in human patients: sublingual capnometry, near-infrared spectroscopy to monitor muscle tissue oxygenation saturation, transcutaneous tissue oxygenation, orthogonal polarization spectral imaging, and capnometry
More invasive techniques: gastric tonometry, which evaluates CO2 production in the stomach wall, infrared spectroscopic assessment of splanchnic perfusion, measurement of bladder mucosal pH
Hypotensive Resuscitation and Delayed Resuscitation
In cases of uncontrolled bleeding, increasing systolic pressure to normal values may dislodge or "blow out" a tenuous clot
Hypotensive resuscitation has been advocated to prevent or minimize further blood loss until surgical control or formation of a stable clot has occurred
Strategies include achieving a MAP of 60 to 65 mmHg using a predetermined, lower fluid infusion rate, or in situations, completely delaying fluid resuscitation until bleeding is surgically controlled
In multiple animal models, controlled resuscitation (goal of MAP 40-60 mmHg or systolic blood pressure of 80-90 mmHg) resulted in decreased blood loss, better splanchnic perfusion and tissue oxygenation, less acidemia, hemodilution, thrombocytopenia, and coagulopathy, decreased apoptotic cell death and tissue injury, and increased survival
Predicting Outcome of Shock
Critical review where high-risk surgical patients were used as a model for shock Nonsurvivors had reduced CO and DO2 in the intraoperative and immediate postoperative period Survivors had lower O2ER, higher hematocrits, VO2, and blood volume, and normal blood gases
Survivors showing fast improvement or normalization of CO, perfusion, oxygen uptake, and clinical variables
Rapid control of hemorrhage, restoration of perfusion, normalization of blood gas values, and prevention of dilutional coagulopathy are predictors of survival
In patients with ongoing blood loss, controlled hypotension has been shown to decrease in-hospital complications and possibly increase survival rates
Lactate, and particularly lactate clearance, has been shown to be strongly associated with survival in both clinical and experimental studies of shock
A poor or absent response to resuscitative attempts with continued evidence of perfusion deficits or the development of clinical evidence of organ dysfunction, or both, are associated with a poorer outcome
Phases of the Metabolic Response to Trauma
Ebb phase
Flow Phase
Ebb Phase of the Metabolic Response to Trauma
Occurs during the first several hours after injury
Characterized by hypovolemia and low flow or perfusion to the injured site
Flow Phase of the Metabolic Response to Trauma
Occurs in the ensuing days to weeks
Begins once perfusion is restored
Catabolic period
- Triggered by mediators involved in the pathophysiology of shock and clinical signs will mimic those seen in shock
Anabolic period
- Characterized by the return to homeostasis
- Cortisol levels fall and normalization of physiology occurs
Mediators fo the Stress Response: the Ebb Phase
Stress response to trauma is initiated by pain, tissue injury, hypovolemia, acidosis, shock, hypothermia, and psychological responses
Direct tissue injury, ischemia, and inflammation activate afferent nerve endings, which exert local and systemic effects via the central nervous system
Hypovolemia, acidosis, and shock exert their effects via baroreceptors and chemoreceptors located in the heart and great vessels
Fear and pain have conscious effects in the cortex, and they stimulate cortisol secretion via the hypothalamic-pituitary-adrenal axis (HPA) which increases sympathetic output Modulation of pain has been shown to be important in controlling the stress response to trauma
The sympathoadrenal axis is stimulated through direct input from injured nerves and by hypovolemia, acidosis, shock, and psychologic responses (fear, pain, anxiety)
Catecholamines have widespread effects on cardiovascular function and metabolism and they stimulate release of other mediators, including cortisol and opioids
Prolonged sympathoadrenal stimulation can be detrimental because of its effects on general body condition Catecholamines increase peripheral vascular resistance so ongoing stimulation leads to long periods of tissue ischemia
Other triggers of cortisol secretion in trauma and shock include AVP, angiotensin II, norepinephrine, and endotoxin
The degree of hypercortisolemia correlates with the severity of injury and persists until the anabolic phase of healing begins
Cortisol secretion results in sodium and water retention (edema) insulin resistance, gluconeogenesis, lipolysis, and protein catabolism
Cortisol also affects leukocytes and inflammatory mediator production
Prolonged cortisol secretion can result in pathologic suppression of the immune system
AVP and RAAS are important mediators of the stress response
Endogenous opioids released from the pituitary gland as well as from the adrenal glands in response to sympathetic stimulation are important mediators in the modulation of pain, catecholamine releases, and insulin secretion Endogenous opioids modulate lymphocyte and neutrophil function and may act to counter cortisol's effect on immune function
Tissue factor exposure activates the coagulation and complement cascades and ultimately stimulates the inflammatory response
Cell membrane injury results in release and activation of the arachidonic acid cascade and production of various cytokines, including prostaglandins, prostacyclines, thromboxanes, and leukotrienes Mediators further activate the coagulation and platelets, alter blood flow via vasoconstriction and vasodilation, and increase chemotactic activity mediating the influx and activation of inflammatory cells with subsequent release of lysosomal enzymes and reactive oxygen species (ROS)
If perfusion is restored, elevated local concentrations of ROS coupled with influx of oxygen, can induce further oxidative stress and production of highly toxic ROS that result in more tissue damage
Amplification of this response coupled with reperfusion can lead to the development of SIRS and MOD
Response to Trauma: Catabolic Period
Many of the changes in vital signs will mimic those seen with hypovolemic shock
Cardiovascular
- Tachycardia
- Tachypnea
- Hypotension
- Decreased perfusion
- Decreased urine output
- Reduced cardiac contractility
Fever
Endotoxemia and bacteremia are likely with gastrointestinal injury, such as strangulating injury to the intestine
Edema at the site of injury is caused by vascular injury from the trauma and the inflammation response, results in loss of capillary integrity and extravasation of protein and fluid
Generalized edema results from systemic inflammatory, hormonal, and autonomic responses that increase capillary pressure and salt and water retention
Decreased appetite and malaise are seen in response to pain, cytokines, and hormones
Coagulation is activated by endothelial injury and the expression of tissue factor Tissue factor also activates complement and inflammation + release of arachidonic acid from damaged cell membranes stimulate production of multiple inflammatory mediators, platelet activation and adhesion, and fibrinolysis
Circulating leukocytes increase in the initial response to injury with subsequent accumulation in injured microvascular beds May be exacerbated by vasoconstriction in response to hypovolemia and catecholamine surges and may play a role in reperfusion injury because activated neutrophils are a major source of reactive oxygen metabolites
Decreases in antibody production, neutrophil chemotaxis, and serum opsonic activity, increases in serum immunosuppressive factors, and activation of T-cell suppressors mediated by neurohormonal stress response
Response to Trauma: Anabolic Period
Appetite returns, body protein is synthesized, and weight is restored
Metabolic demands diminish, water balance is restored, and as hormonal levels decrease, a generalized feeling of well-being develops