Chem 001b Order Concen vs Time and Activation Energy
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Last updated 1:52 AM on 9/9/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
1
New cards
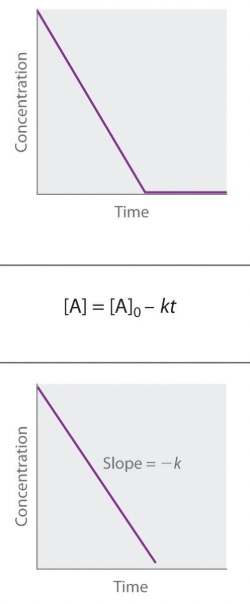
Zero Order
Concentration vs time
Integrated Rate Law
Determine Rate Constant
2
New cards
First Order
Concentration vs time
Integrated Rate Law | ln [a]t/[b]o = -kt
Or ln[a]t=-kt + ln[a]o
Determine Rate Constant
![<ol><li><p>Concentration vs time</p></li><li><p>Integrated Rate Law | ln [a]t/[b]o = -kt</p></li></ol><p>Or ln[a]t=-kt + ln[a]o</p><ol><li><p>Determine Rate Constant</p></li></ol><p></p>](https://knowt-user-attachments.s3.amazonaws.com/a01c30ff-5cd0-49e5-a343-70fd343c72b4.png)
3
New cards
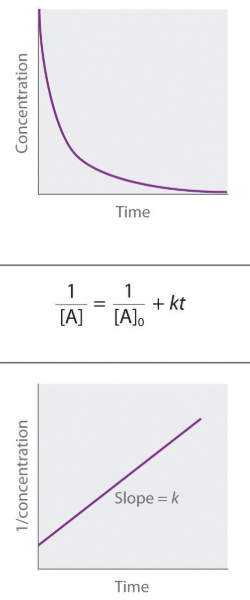
Second Order
Concentration vs time
Integrated Rate Law
Determine Rate Constant
4
New cards
Activation Energy Formula (Solve for k)
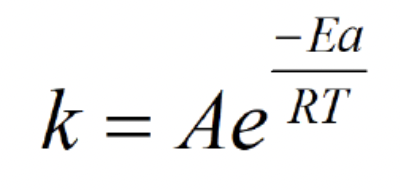
5
New cards
Arrhenius Equation
ln(k2/k1) = Ea/R * (1/T1 - 1/T2)
6
New cards
R Constant
R = 8.314