PHYSICS - PROPERTIES OF MATTER - Specific latent heat
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
change if state
physical change where a substance changes from 1 state to another without a change in chemical composition
During change of state:
Temperature remains constant
energy is either absorbed or released
energy is used to break or form intermolecular bonds, not increase kinetic energy
melting
solid to liquid
freezing
liquid to solid
evaporation
liquid to gas
condensation
gas to liquid
sublimation
solid to gas
specific latent heat (L)
amount of heat energy required to change the state of 1kg of a substance without change of temperature
J/kg
specific latent heat if fusion (Lf)
energy required to change 1kg if a substance from solid to liquid at a constant temperature
specific latent heat of vapourisation (Lv)
energy required to change 1kg of a substance from liquid to gas at a constant temperature
formula of SLH
E = mL
no temperature change when heat energy is added or removed during state change
temperature remains constant until change is complete
energy is used to overcome forces of attraction between particles in a substance
different substances have different Lf and Lv
substances with strong intermolecular forces have higher L as more energy is required to change their state
energy conservation
total energy gained by another substance = total energy lost by another substance
practical applications of SLH
melting and freezing - ice absorbs a lot of energy as it melts without changing temperature, useful of cooling drinks and preserving food
boiling and condensation - steam releases a lot of energy when it condenses, steam burns more severe than burns from water
refrigeration - fridges remove heat from food using SLH, refrigerants absorb heat during evaporation and release it during condensation
cooling curve
a graph that shows how the temperature of a substance changes over time as it loses heat
used to analyse the cooling and phase changes of a substance
key features of a cooling curve
cooling in gas phase - temperature of gas decreases as it loses heat, particles lose kinetic energy, resulting in reduced temperature
condensation - during condensation, temperature remains constant even though heat is beung released, as energy is being used to form intermolecular bonds as gas becomes liquid
cooling in liquid phase - temperature of liquid decreases as it loses heat, particles move slower as their kinetic energy decreases
freezing - energy used to form stronger intermolecular bonds as liquid becomes solid
cooling in solid phase - temperature of solid decreases as it loses heat, particles vibrate less as their kinetic energy decreases
cooling curve interpretation
flat sections represent state changes
sloped sections represent temperature changes
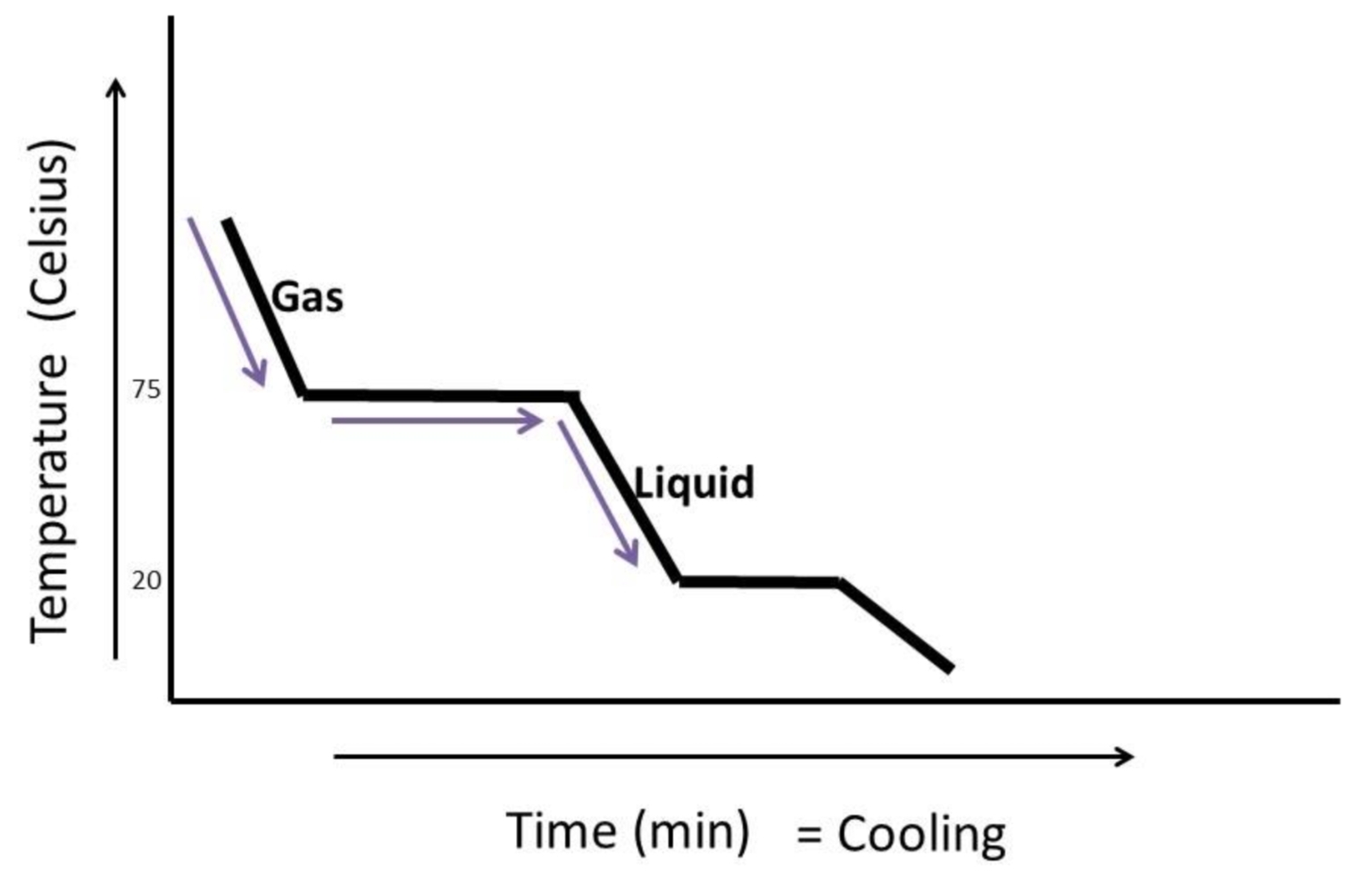
SLH and cooling curves
flat sections of curve represent energy involved in a change of state
latent heat of fusion - occurs during freezing
latent heat of vapourisation - occurs during condensation