module 11: other gram negative rods
1/61
Earn XP
Description and Tags
those not in the enterobacteriaceae family
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
62 Terms
vibrio cholerae
causes epidemic Asiatic cholera. human is the only host and source as they shed this bacteria in feces, contaminating food & water supplies. 2 strains/biotypes distinguished by phenotypic markers & O (somatic) antigens (both have O1).
vibrio cholerae: strain El Tor
milder form of the disease, patient can be asymptomatic. bacteria survives in the body longer than the other strain; allows carriers to infect more people in the population. responsible for most global cholera cases. biotype that caused cholera in Haiti after earthquake
vibrio cholerae: classical
cholera that’s relatively rare globally except in India & Bangladesh
convalescent carriers
those recovering from disease who shed bacteria for up to a year
chronic carriers
those who no longer have disease but still carry bacteria for years. store bacteria in the gall bladder → gallbladder intermittently sheds bacteria, which can make ID of individuals difficult
how is vibrio cholerae transmitted?
fecal-oral route: ingestion of contaminated food or water. ex: fruits & vegetables grown in sewage-rich soil that are ingested without prior cooking, eating raw oysters. after ingestion, bacteria multiply rapidly in the intestine
pathogenesis of vibrio cholerae
bacteria produce enterotoxin that acts on intestinal lining, causing massive fluid & electrolyte loss (up to 20 L/day). voided fluid contains high electrolytes. onset of symptoms abrupt with severe vomiting & diarrhea: “rice-water stools”
complications from vibrio cholerae
result from massive loss of essential electrolytes. hypovolemic shock & metabolic acidosis. untreated mortality rate ~50%. good news: immunity is long-lasting for most people after resolution
how to prevent vibrio cholerae?
desiccation, sunlight, & acid kill this, but microbial dose is so great that losing some bacteria to stomach acid is not sufficient. single dose vaccines available. oral cholera vaccine (OCV), but limited protection in children. proper sanitation & hygiene
how to treat vibrio cholerae?
fluid & electrolyte management: rehydration 1st priority. antibiotics such as tetracycline, which lower bacteria load → lowers enterotoxin released. also eliminates [bacteria] in gall bladder bile → eliminating carrier state
laboratory diagnosis of vibrio cholerae
gram negative rod, comma-shaped. growth on simple culture/agar; media specific for this is also available. oxidase positive (enterics are negative). lactose negative
campylobacter
causes 5-11% of all diarrhea cases in the USA. most human illness caused by jejuni. all cases occur as isolated, sporadic events (not large outbreaks). many causes undiagnosed or unreported. affects 1M people annually
campylobacter jejuni
source is lower animals (dogs, cats), but birds carry it without getting sick; people acquire this from infected stool of sick pet. invasive & infiltrates lining of intestine: causes fever, cramps, bloody diarrhea, ulceration at mucosal surface of intestine. self-limiting, treated with erythromycin
how is campylobacter jejuni transmitted?
handling raw poultry or eating raw/undercooked poultry meat, unpasteurized dairy products, contaminated water. freezing reduces bacteria present on raw meat. very small amount of organisms can cause illness in humans (one drop of raw chicken juice)
environmental reservoirs that can lead to human infection of C. jejuni
colonization of GI tract of chickens: bacteria passes through chicks in a flock through fecal-oral route → humans consume contaminated animal products. can enter water supply & infect humans via drinking water. invades epithelium of human intestine: inflammation, diarrhea follow

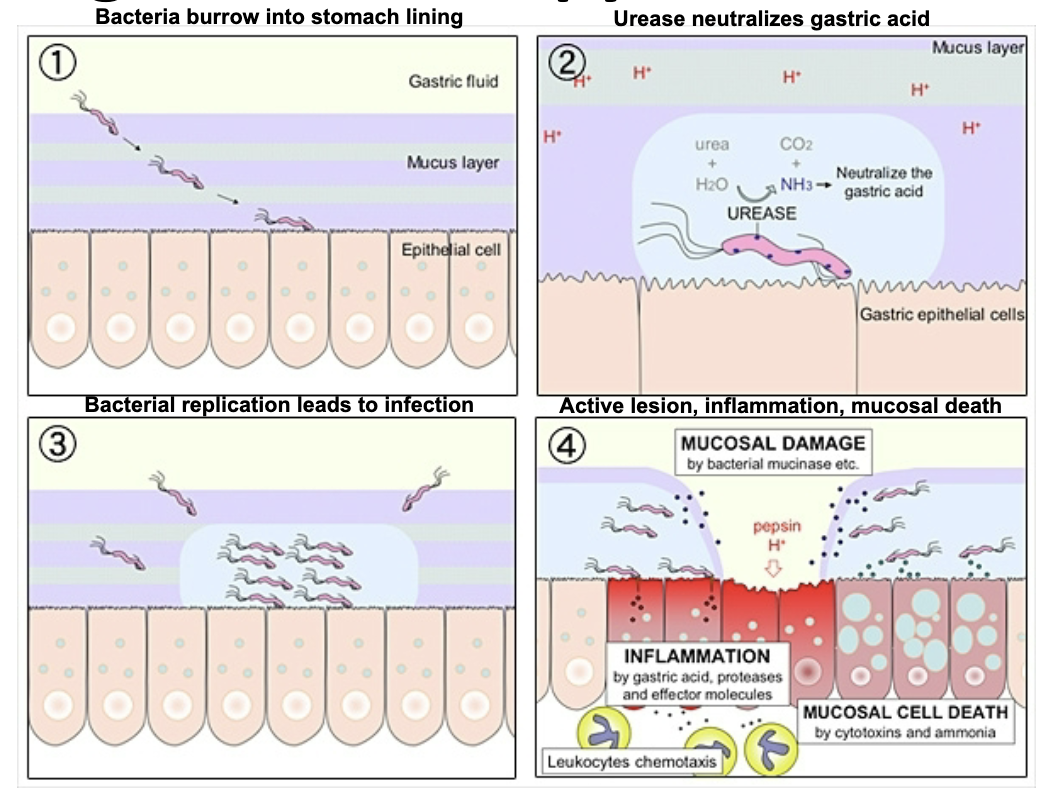
helicobacter pylori
leading cause of peptic ulcers and chronic gastritis in the USA. pathogenesis mechanisms include motility, urease activity, & association with gastric mucosal cells (important virulence factors). long-term infection leads to risk of gastric cancer & MALT lymphoma
source of helicobacter pylori
more than 50% of the population globally harbors this in upper GI tract. most infected people asymptomatic. many people infected as children. transmitted person-to-person with direct contact (saliva, vomit, feces) or fecal contamination of food/water
endoscopy & associated biopsy for H. pylori
reference method for diagnosis. specimens of stomach & duodenum obtained. use urease test on tissue (this bacteria is urease positive). use histologic stain of tissue & culture to ID bacteria in tissue (gold standard of diagnostic tests)

urea breath test for H. pylori
patient given 14C-labeled urea to drink. the bacteria metabolizes urea rapidly & labeled carbon is released. labeled carbon measured in patient’s breath to determine whether bacteria is present
serum antibody test for H. pylori
measure specific IgG antibodies having reactivity against this bacteria. if antibodies present, then patient has or had the infection
stool antigen test for H. pylori
accurate, noninvasive test for direct detection of this bacteria in stools. antigen detection = active infection. definitive test of choice for diagnosis & treatment monitoring
helicobacter pylori treatments
antibiotics like amoxicillin & tetracycline. antacid or proton pump inhibitor: help alleviate ulcer-related symptoms, heal gastric mucosal inflammation
laboratory diagnosis for campylobacter & helicobacter
gram negative rods, curved & S-shaped. no growth on blood or MacConkey agars. microaerophilic: requires high CO2 (killed by O2 content in air). temp preference 42C (higher than most bacteria). nonfermentative, oxidase positive, catalase positive
pseudomonas
found in soil, water as natural habitat. most don’t infect man, but those that do cause severe infections and are difficult to treat.
pseudomonas aeruginosa
found nearly everywhere and may be harbored in nearly any site in medical environment (water systems): contaminated equipment like IV fluids & water. easily spread from patient to patient via hospital personnel. infections usually occur in people with altered host defenses. resistant to many antibiotics
pseudomonas aeruginosa laboratory diagnosis
aerobic gram negative rods. nonfermentative, don’t utilize glucose by fermentation. most frequently isolated nonfermenter in clinical setting. produces pigments pyocyanin & fluorescein: blue-green, fluorescent under UV light, occurs in virtro & in vivo
pyocyanin
kills competing microbes. generates reactive oxygen species such as H2O2 & superoxide anion. inactivates catalase. interferes with electron transport chain
clinical infections of P. aeruginosa
lesions may spread via bloodstream causing septicemia. localized lesions may occur in burns, wounds, corneal tissue, lungs, urinary tract. eye infections. highly susceptible states: leukemia patients, burn patients, cystic fibrosis patients (lung infections). can be prevented by heptavalent vaccine
treatment of P. aeruginosa
topical antimicrobics for burns, wounds, eyes; systemic by ingestion (enteral) or injection (parenteral → not thru GI tract). systemic consists of combination strategy. aminoglycosides & penicillins
burkholderia pseudomallei
responsible for meliodosis (Whitmore’s disease), which is characterized by pneumonia & multiple abscesses. mortality rate 40%. found in soil & water in tropical areas of Southeast Asia. 5-20% agricultural workers have antibody to it. can remain latent for a long time: relapse known to occur
burkholderia pseudomallei disease presentation
ingestion or inhalation of contaminated dust, soil contamination of abraded skin. infection where abscess forms and leads to aggressive granulomatous disease. further abscess forms in lungs & other viscera. overwhelming & rapidly fatal septicemia can occur
hemophilus
small gram negative rods (coccobacilli). characterized by a requirement for specific growth factors that are found in blood. species of importance: influenzae, aegyptius, ducreyi, gardnerella vaginalis (vaginale)
hemophilus influenzae
source: humans, 30% of adults have it in upper respiratory tract. transmitted human to human by inhalation of infected droplets from active cases or carriers. causes upper & lower respiratory tract infections (pneumonia), meningitis (most serious), & acute bacterial epiglottitis (lead to closed off airways, sepicemia)
laboratory diagnostics for hemophilus influenzae
chocolate agar: V factor (NAD) & X factor (hemin). serotyped into 6 groups utilizing capsular antigens A → F. type B is most common cause of acute bacterial meningitis in infants/children. non-epidemic
treatment & prevention of H. influenzae
antibiotics: ampicillin & chloramphenicol. since it’s resistant to phagocytosis by alveolar macrophages, several vaccines are available (part of routine childhood vaccination)
hemophilus aegypitus: Koch-Weeks Bacillus
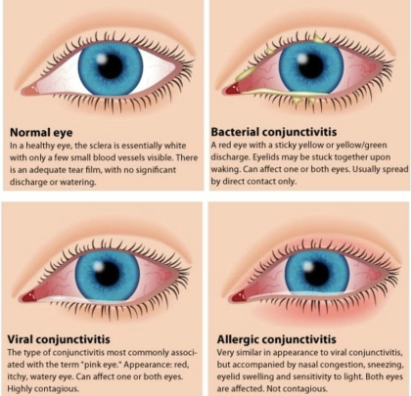
needs to be differentiated serologically from H. influenzae. causes conjunctivitis (pink eye), which can be epidemic in kids. treated with local administration of ophthalmic antibiotic solution
hemophilus ducreyi: Chancroid Bacillus
venereal disease. initial infection causes formation of soft chancre, a painful ulcerative sore on genitalia that bleeds easily if scraped. transmitted by direct contact, highly contagious
hemophilus vaginale (gardnerella vaginalis)
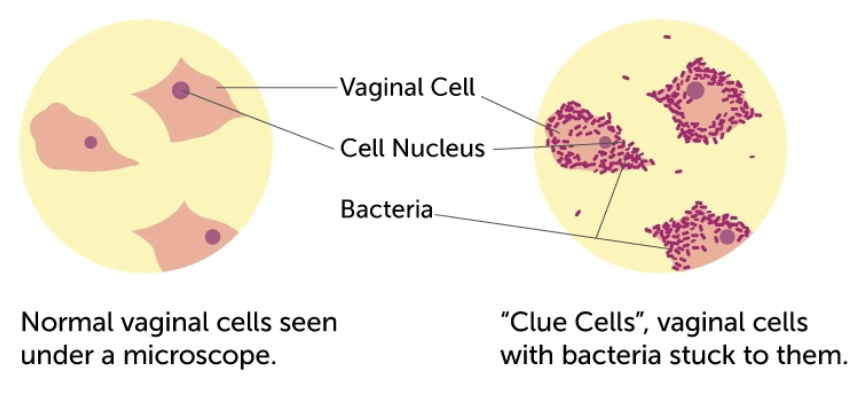
venereal transmission that is associated with vaginitis. does not invade tissue, but grows in vaginal secretions. ID by clue cells, which are squamous epithelial cells with adhered masses of gram negative pleomorphic rods
bordetella pertussis
causative agent of whooping cough in humans. usually children with disease. healthy adults are reservoirs. can be misdiagnosed. transmitted by inhalation or direct contact with discharges from respiratory mucous membranes of people infected. coughing aerosolizes bacteria, transmitting it to susceptible individuals
laboratory diagnosis of bordetella pertussis
strict aerobe. requires bordet-gengou medium (potato blood agar). slow grower, so use PCR or fluorescent antibody test. contains capsular K antigens, but serologic testing is difficult to interpret
pertussis (whooping cough)
organism aggregates on bronchial & tracheal lining and toxins are released: exotoxin causes bacteria to adhere to cells, cytotoxicity & cell necrosis. 3 stages: catarrhal, paroxysmal, & convalescence. can lead to CNS disorders and secondary infections in ears, sinuses, respiratory tract
catarrhal (stage 1)
most highly infectious period. upper respiratory involvement. mild cold-like symptoms like coughing, sneezing, slight fever, runny nose.
paroxysmal (stage 2)
cough described as paroxysmal (sudden intensification of symptoms) or spasmodic (sudden but transitory airway construction). coughs so forceful & close that patient can’t breath. at the end of spasm, gasp for air which sounds like a whoop. young infants don’t whoop, which is bad
convalescence (stage 3)
less severe and less frequent paroxysms
treatment & prevention of bordetella pertussis
antibiotic erythromycin. fluid & electrolyte management, oxygen therapy to avoid anoxia. DTap vaccine and TDap booster (for diptheria, tentanus, pertussis)
brucella
bacteria that are intracellular parasites that infect lower animals and are transmissible to man. causes contagious abortion in lower animals (abortus). causes brucellosis (undulant fever) in humans, which rises & falls like a wave (melitensis). worldwide, concentrated in Mexico, Africa, India, & Europe
brucella melitensis
from infected animals or secretions (milk), animals may recover quickly but excrete this for varying lengths of time. transmitted by ingestion of contaminated milk, consumption of undercooked meat, direct contact with sick animals, or inhalation (rare)
how does brucella melitensis initiate infection?
it enters through skin (direct contact) or are ingested. disseminate through lymphatics & bloodstream. remain intact within phagocytes (especially macrophages) where they’re protected from antibiotics. may cause abscess formation in infected tissue. localize in spleen, bone marrow
symptoms of brucella melitensis
nonspecific manifestations like weakness, chills, malaise, headache. intermittent (undulant) fever due to endotoxin release. sometimes mental depression & increased nervousness. can last a while. complications: arthritis, endocarditis, neurologic disorders
laboratory diagnosis of brucella melitensis
found intracellular in phagocytes or tissue. slow growing. BSL-3. serological test for antibody is recommended. febrile agglutination rapid results. panel of tests that rule in/out different diseases. specific agglutination tests for this. acute & convalescent serum samples are norm for diagnosis

treatment & prevention of brucellosis (malta, crimean, gibraltar fever)
antibiotics: tetracycline &/or rifampin, chloramphenicol. intracellular localization of bacteria may contribute to antimicrobic inefficiency/ineffectiveness. control animal infections (& meat from infected animals), pasteurize milk & milk products
francisella tularensis
found intracellular in macrophages. causative agent of tularemia (rabbit fever). acute infectious disease of wild animals, especially rabbits & ground squirrels. direct contact is most common mode of transmission, also bites of insect vectors. febrile disease, chills, headaches, back pain, progressive weakness, exhaustion
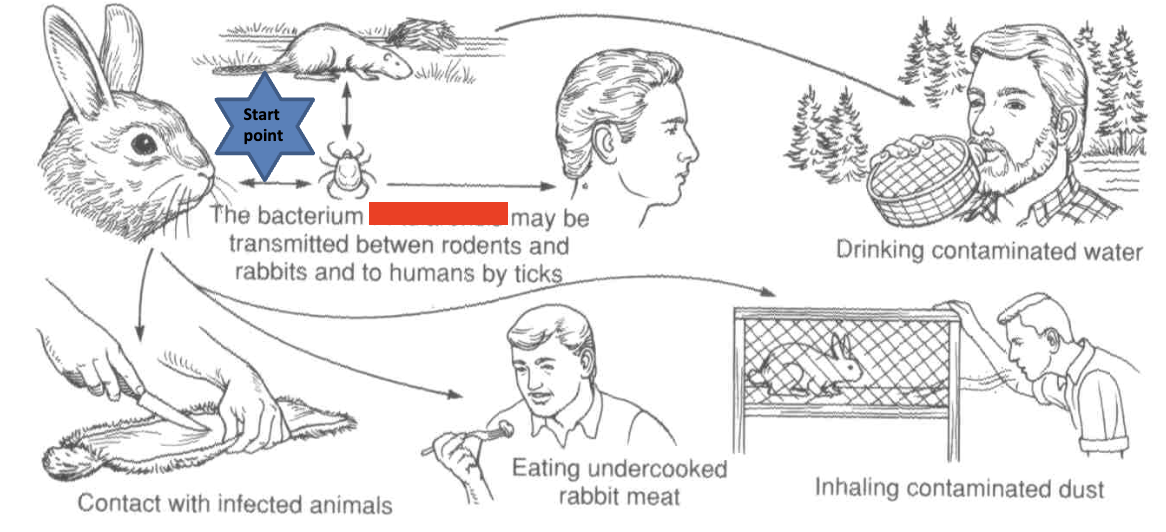
disease progression of F. tularensis
sequesters in phagosome of macrophages, breaks out into cytosol where it rapidly proliferates. infected macrophages undergoes apoptosis, releasing bacteria that initiate more infections. infected lymph glands drain into lymph nodes where bacteria enters blood stream. cycle starts again: phagocytes in bloodstream ingest bacteria
clinical forms of tularemia
ulceroglandular (direct contact), oropharyngeal infection (can be due to ingestion), oculoglandular (direct contact), glandular (direct contact, no ulcers), pneumonic (aerosol inhalation), septicemic, typhoidal
ulceroglandular tularemia
most cases. direct contact. cutaneous ulcer at site of infection. regional lymph nodes become swollen & painful (lymphadenopathy) due to bacterial transport by macrophages
occuloglandular tularemia
purulent conjunctivitis. similar to ulceroglandular except conjunctiva is primary site of infection. usually results from rubbing eyes with contaminated fingers
typhodial tularemia
focus of infection is mouth, throat, GI tract. systemic illness with fever with toxemia in liver, spleen. can be due to ingestion of bacteria
laboratory diagnosis of F. tularensis
PCR. growth on blood & chocolate agar (fastidious). specimen is symptom-dependent. serological tests most common. elevated antibody titer to antigen in patient w/o history of vaccination in conjunction with symptoms. ELISA or agglutination rxns
treatment & prevention of F. tularensis
antibiotics: streptomycin. avoid infected animals, wash hands, cook food thoroughly, drink safe water, insect repellent, vaccinated if high exposure
pasteurella multocida
transmitted from animal to man via animal licks, bites, or scratches (dogs/cats). cellulitis & abscess formation at the site. complications: sepsis, meningitis, osteomyelitis. specimen: exudate from lesion, blood if fever. culture & biochem ID. clean wound, avoid suturing. initiate antibiotics (penicillin, tetracycline) ASAP
legionella pneumophila
from water supplies (water holding vats), especially with biofilms, has tolerance for chlorine. inhalation of infected water droplets (aerosolized particles). upper respiratory tract infections. can be asymptomatic. associated with legionnaires disease (fever, pneumonia) & pontiac fever (milder w/ no pneumonia)
laboratory diagnosis of legionella pneumophila
gram negative rods. able to enter, survive, & multiply within host cells, especially macrophages & neutrophils. treated with erythromycin or erythromycin/rifampin combo.