Structure and Properties of Crystals + Allotropes of Carbon
0.0(0)
Card Sorting
1/10
Earn XP
Description and Tags
Last updated 5:23 PM on 7/16/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
1
New cards
crystalline solids
Atoms, ions, or molecules that are arranged in a regular repeating pattern is called a **crystal lattice**. Crystals can be classified as: **Ionic solids, Molecular solids, Metallic solids,** or **Covalent Network solids**
2
New cards
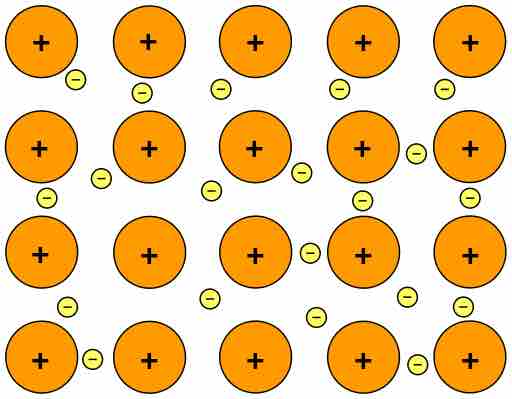
ionic crystals
**Structural Particles:** Alternating cations (+ve) and anions (-ve)
**Intramolecular Force:** Ionic bonds (strong, __directional__)
**Intermolecular Force:** ION-ION electrostatic force
* __Hardness__: Very hard, resists scratching and denting
* __Melting Point__: Moderate to very high (\~1000°C)
* __Electrical Conductivity__: NO as solid; YES when aqueous and molten
* __Solubility__: NO in oil; MANY in water → mobbed
* __Brittleness__: **Very brittle**, easily broken apart (like charges come into contact, repel and break apart)
**Intramolecular Force:** Ionic bonds (strong, __directional__)
**Intermolecular Force:** ION-ION electrostatic force
* __Hardness__: Very hard, resists scratching and denting
* __Melting Point__: Moderate to very high (\~1000°C)
* __Electrical Conductivity__: NO as solid; YES when aqueous and molten
* __Solubility__: NO in oil; MANY in water → mobbed
* __Brittleness__: **Very brittle**, easily broken apart (like charges come into contact, repel and break apart)
3
New cards
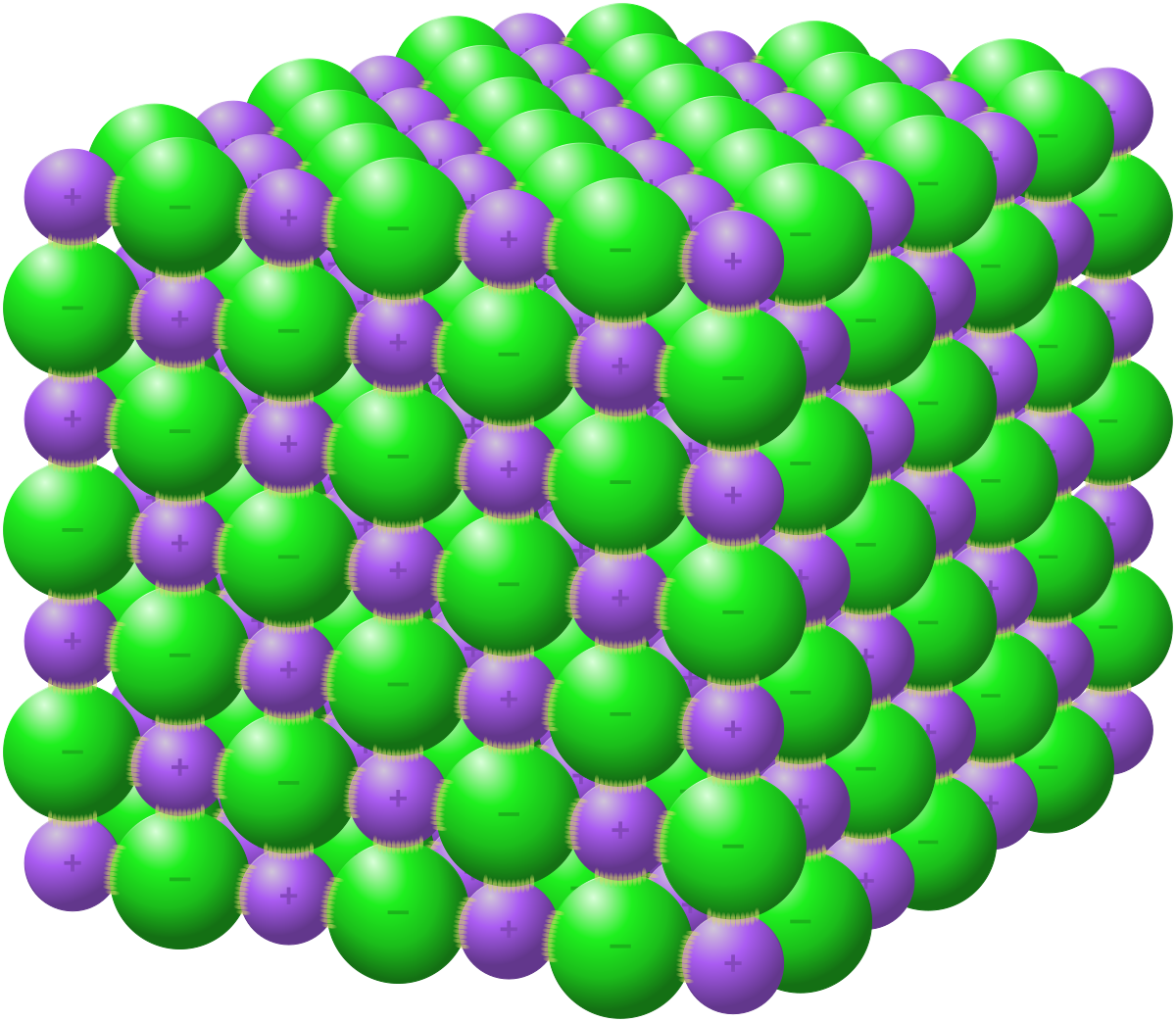
metallic crystals
**Structural Particles:** cation “islands” in a “sea” of delocalised electrons
**Intramolecular Force:** Metallic bonds (strong, __non-directional__ - acts in all directions)
**Intermolecular Force:** cation-electron electrostatic force (strong - __non-directional__)
* __Hardness:__ Varies from soft to very hard (stronger electrostatic forces and compactness means cations can be held in place more firmly and resist sliding over each other - the more delocalised electrons, the more it resists denting and scratching)
* __Melting Point:__ Varies from low to high depending on hardness (strong electrostatic forces resist separation)
__Electrical Conductivity:__ **Very good conductors** both in solid and liquid state (molten), __***won’t conduct in water because it won’t dissolve***__
* __Solubility:__ Not soluble (overall neutral, so nothing to establish forces with the solvent)
* Luster: shiny
* __Malleability/Ductility:__ **Malleable** (can be hammered) and **ductile** (can be drawn into wires) → cations __**slide**__ past each other without coming into contact, hence not breaking the bond
**Intramolecular Force:** Metallic bonds (strong, __non-directional__ - acts in all directions)
**Intermolecular Force:** cation-electron electrostatic force (strong - __non-directional__)
* __Hardness:__ Varies from soft to very hard (stronger electrostatic forces and compactness means cations can be held in place more firmly and resist sliding over each other - the more delocalised electrons, the more it resists denting and scratching)
* __Melting Point:__ Varies from low to high depending on hardness (strong electrostatic forces resist separation)
__Electrical Conductivity:__ **Very good conductors** both in solid and liquid state (molten), __***won’t conduct in water because it won’t dissolve***__
* __Solubility:__ Not soluble (overall neutral, so nothing to establish forces with the solvent)
* Luster: shiny
* __Malleability/Ductility:__ **Malleable** (can be hammered) and **ductile** (can be drawn into wires) → cations __**slide**__ past each other without coming into contact, hence not breaking the bond
4
New cards
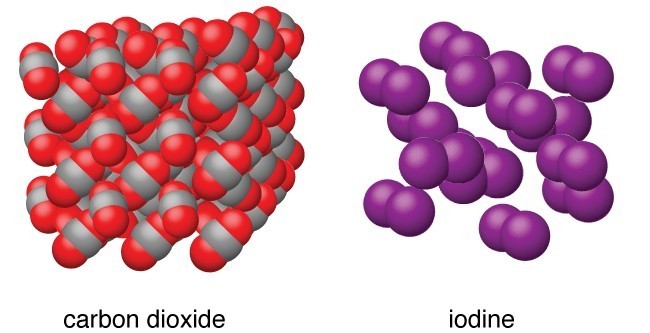
molecular crystals
\*\* biggest family!
**Structural Particles:** Atoms, nonpolar molecules, polar molecules, or molecules H-bonded to F, O, N
**Intramolecular Force:** Polar or nonpolar covalent bonds (strong, __directional__ force within molecules)
**Intermolecular Force:** London Dispersion < Dipole-Dipole < Hydrogen bonding (weak, __somewhat directional__)
* __Hardness:__ Soft (weak intermolecular forces + loose messy lattice mean they are “manipulative” and “squishy”)
* __Melting Point:__ Low to moderate, can evaporate directly, most melt below 300°C (weak intermolecular forces → more easily separated; MP of nonpolar solids increases with molar mass, MP of polar molecular solids increases with strength of IMF)
* __Electrical Conductivity:__ POOR, they are __**insulators**__ (electrons are shared and thus locked in covalent bonds so no ions or electrons can flow → no mobile electrons)
* __Solubility:__ “**Like dissolves Like”**; in oil YES if NP; in water YES if P
* __Brittleness:__ Low flexibility (weak, loose, messy lattice crumbles under pressure)
**Structural Particles:** Atoms, nonpolar molecules, polar molecules, or molecules H-bonded to F, O, N
**Intramolecular Force:** Polar or nonpolar covalent bonds (strong, __directional__ force within molecules)
**Intermolecular Force:** London Dispersion < Dipole-Dipole < Hydrogen bonding (weak, __somewhat directional__)
* __Hardness:__ Soft (weak intermolecular forces + loose messy lattice mean they are “manipulative” and “squishy”)
* __Melting Point:__ Low to moderate, can evaporate directly, most melt below 300°C (weak intermolecular forces → more easily separated; MP of nonpolar solids increases with molar mass, MP of polar molecular solids increases with strength of IMF)
* __Electrical Conductivity:__ POOR, they are __**insulators**__ (electrons are shared and thus locked in covalent bonds so no ions or electrons can flow → no mobile electrons)
* __Solubility:__ “**Like dissolves Like”**; in oil YES if NP; in water YES if P
* __Brittleness:__ Low flexibility (weak, loose, messy lattice crumbles under pressure)
5
New cards
covalent network crystals
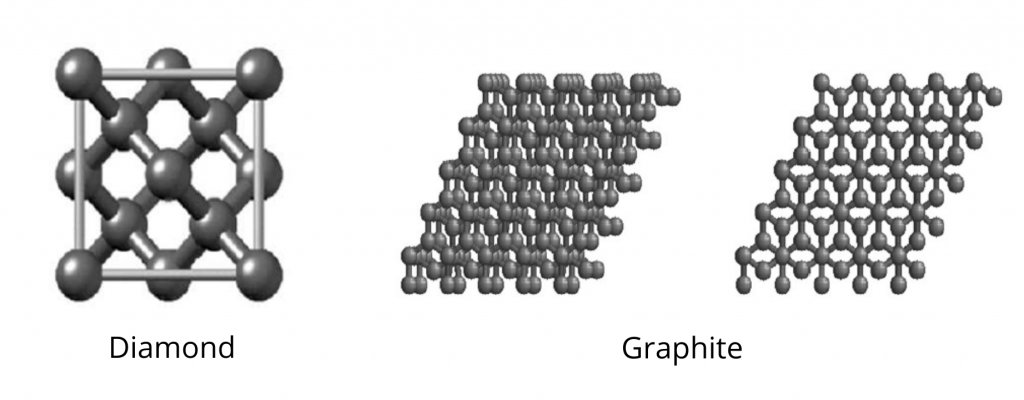
\*\* e.g. sand, quartz, diamond, graphite
Formed by __very many atoms__ or __molecules__ into ONE GIANT MOLECULE
**Structural Particles:** Atoms (C - diamond, graphite, buckyballs; SiC - moissanite; SiO2 - quartz, sand)
**Intramolecular Force:** Covalent bonds (strong, __directional__, interlocking)
**Intermolecular Force:** London Dispersion forces - *in some cases*
Formed by __very many atoms__ or __molecules__ into ONE GIANT MOLECULE
**Structural Particles:** Atoms (C - diamond, graphite, buckyballs; SiC - moissanite; SiO2 - quartz, sand)
**Intramolecular Force:** Covalent bonds (strong, __directional__, interlocking)
**Intermolecular Force:** London Dispersion forces - *in some cases*
6
New cards
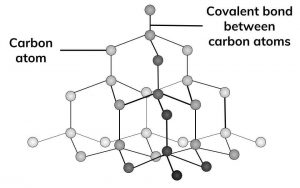
diamond
**Covalent Network Crystal**, tetrahedral (giant crystal lattice), sp3 hybridization and sigma bonds, no IMFs, VERY HARD (10 Mohs)
* __Hardness:__ Very hard (strong direction covalent bonds and tetrahedral network resists denting and scratching)
* __Melting Point:__ **Sublimes** at 4027°C (strong covalent bonds and tetrahedral network resists separation)
* __Electrical Conductivity:__ **No** (electrons shared in bonds so no ions or electrons can flow)
* __Solubility:__ **No** (overall neutral, nothing to establish forces with the solvent)
* __Malleability/Ductility:__ **No** (complex interlocking network resists flexibility and will break under pressure along fault lines)
* __Hardness:__ Very hard (strong direction covalent bonds and tetrahedral network resists denting and scratching)
* __Melting Point:__ **Sublimes** at 4027°C (strong covalent bonds and tetrahedral network resists separation)
* __Electrical Conductivity:__ **No** (electrons shared in bonds so no ions or electrons can flow)
* __Solubility:__ **No** (overall neutral, nothing to establish forces with the solvent)
* __Malleability/Ductility:__ **No** (complex interlocking network resists flexibility and will break under pressure along fault lines)
7
New cards
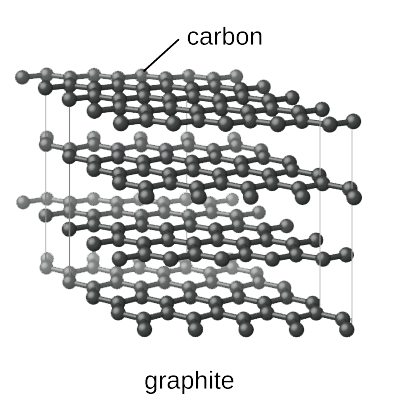
graphite
**Covalent Network Crystal**, trigonal planar (layer-like structure, atoms arranged in flat layers of hexagons, between which is a __soup__ of free-floating, delocalised electrons made up of the spare electrons from sp2 hybridization → pi cloud)
* __Hardness:__ Soft (2-D sheets/layers of hexagonal rings of carbon atoms slide past each other)
* __Melting Point:__ **Sublimes** at 3600°C (London Dispersion forces between sheets resist separation __due to size__)
* __Electrical Conductivity:__ **YES!!** (Pi-cloud has mobile electrons)
* __Solubility:__ **No** (overall neutral, nothing to establish forces with the solvent)
* __Malleability/Ductility:__ **FLAKEY** (2-D sheets __slide__ past each other; 1-2 Mohs)
* __Hardness:__ Soft (2-D sheets/layers of hexagonal rings of carbon atoms slide past each other)
* __Melting Point:__ **Sublimes** at 3600°C (London Dispersion forces between sheets resist separation __due to size__)
* __Electrical Conductivity:__ **YES!!** (Pi-cloud has mobile electrons)
* __Solubility:__ **No** (overall neutral, nothing to establish forces with the solvent)
* __Malleability/Ductility:__ **FLAKEY** (2-D sheets __slide__ past each other; 1-2 Mohs)
8
New cards
allotrope
Each of two or more different physical forms in which an element can exist; differ in the arrangement of atoms in crystalline solids or in the occurrence of molecules that contain different numbers of atoms.
9
New cards
catenation
The bonding of atoms of the same element into a series, called a chain.
10
New cards
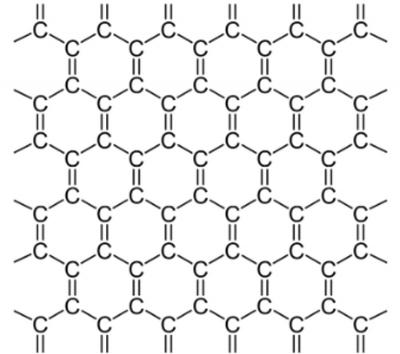
graphene
**Covalent Network Crystal**, trigonal planar (thinnest, lightest, strongest, most stretchy material we have ever created, think of it like a single layer extracted from graphite)
* Hexagonal lattice
* __Hybridization:__ sp2 hybridization
* __IMFs?:__ London Dispersion forces
* __Conductivity__: Excellent conductor (300x more efficient than copper) due to pi-cloud formed by unhybridized p orbitals
* __Melting Point:__ Sublimes at 3625°C
* __Solubility__: No solubility in water
* __Hardness:__ Very strong (>10 Mohs)
* Hexagonal lattice
* __Hybridization:__ sp2 hybridization
* __IMFs?:__ London Dispersion forces
* __Conductivity__: Excellent conductor (300x more efficient than copper) due to pi-cloud formed by unhybridized p orbitals
* __Melting Point:__ Sublimes at 3625°C
* __Solubility__: No solubility in water
* __Hardness:__ Very strong (>10 Mohs)
11
New cards
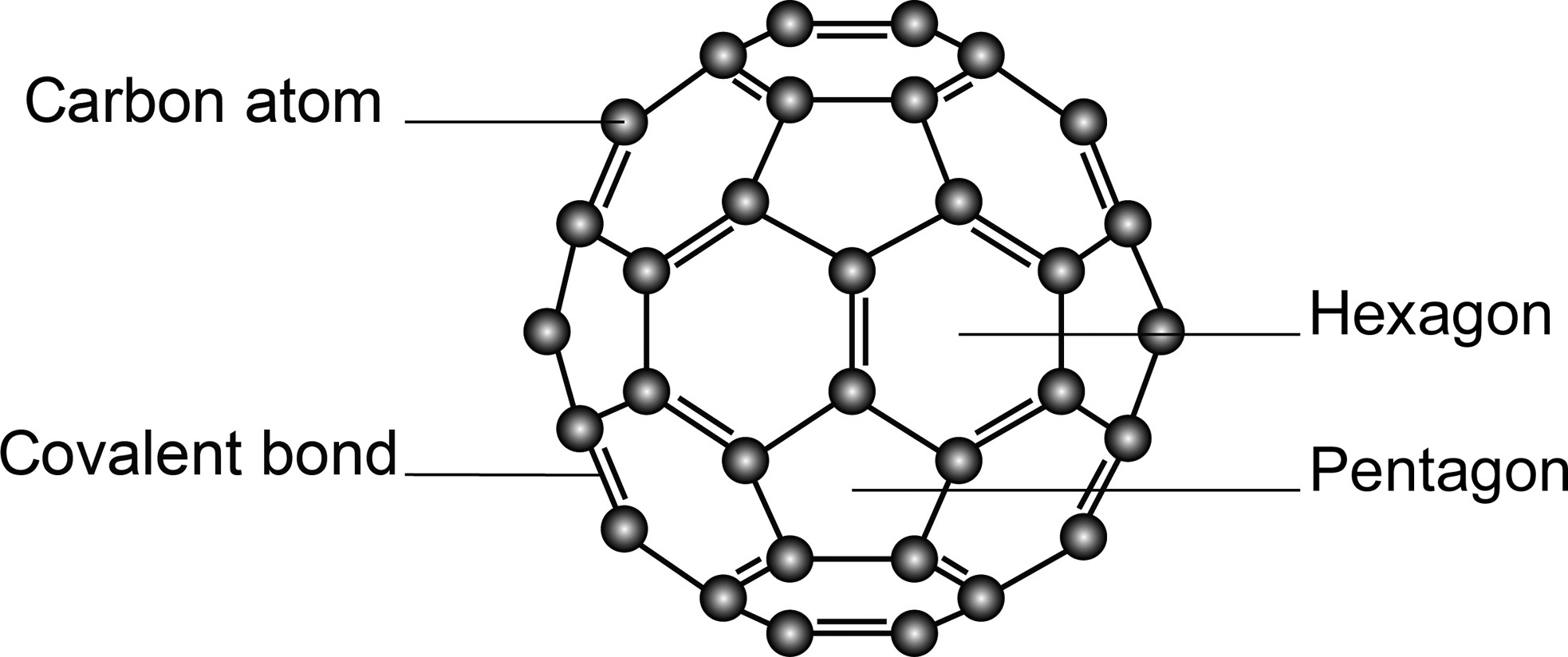
C60 Fullerene
**Molecular Crystal** (small, only 60 carbons), trigonal planar (60 C arranged as 10 hexagons and 12 pentagons like a soccer ball)
* __Hybridization__: sp2 hybridization
* __IMFs?:__ Weak London Dispersion forces between C60 molecules
* __Conductivity:__ Poor conductor compared to graphite; fewer delocalised electrons capable of moving from one molecule to the next
* __Melting Point__: Sublimes at 600°C (IMFs are weaker, thus, MP is low)
* __Solubility:__ No solubility in water
* __Hardness:__ Very strong (>10 Mohs)
* __Hybridization__: sp2 hybridization
* __IMFs?:__ Weak London Dispersion forces between C60 molecules
* __Conductivity:__ Poor conductor compared to graphite; fewer delocalised electrons capable of moving from one molecule to the next
* __Melting Point__: Sublimes at 600°C (IMFs are weaker, thus, MP is low)
* __Solubility:__ No solubility in water
* __Hardness:__ Very strong (>10 Mohs)