measuring electrode potentials and cell notation
1/12
Earn XP
Description and Tags
update w/ tricky examples and h2so4 conc thing
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
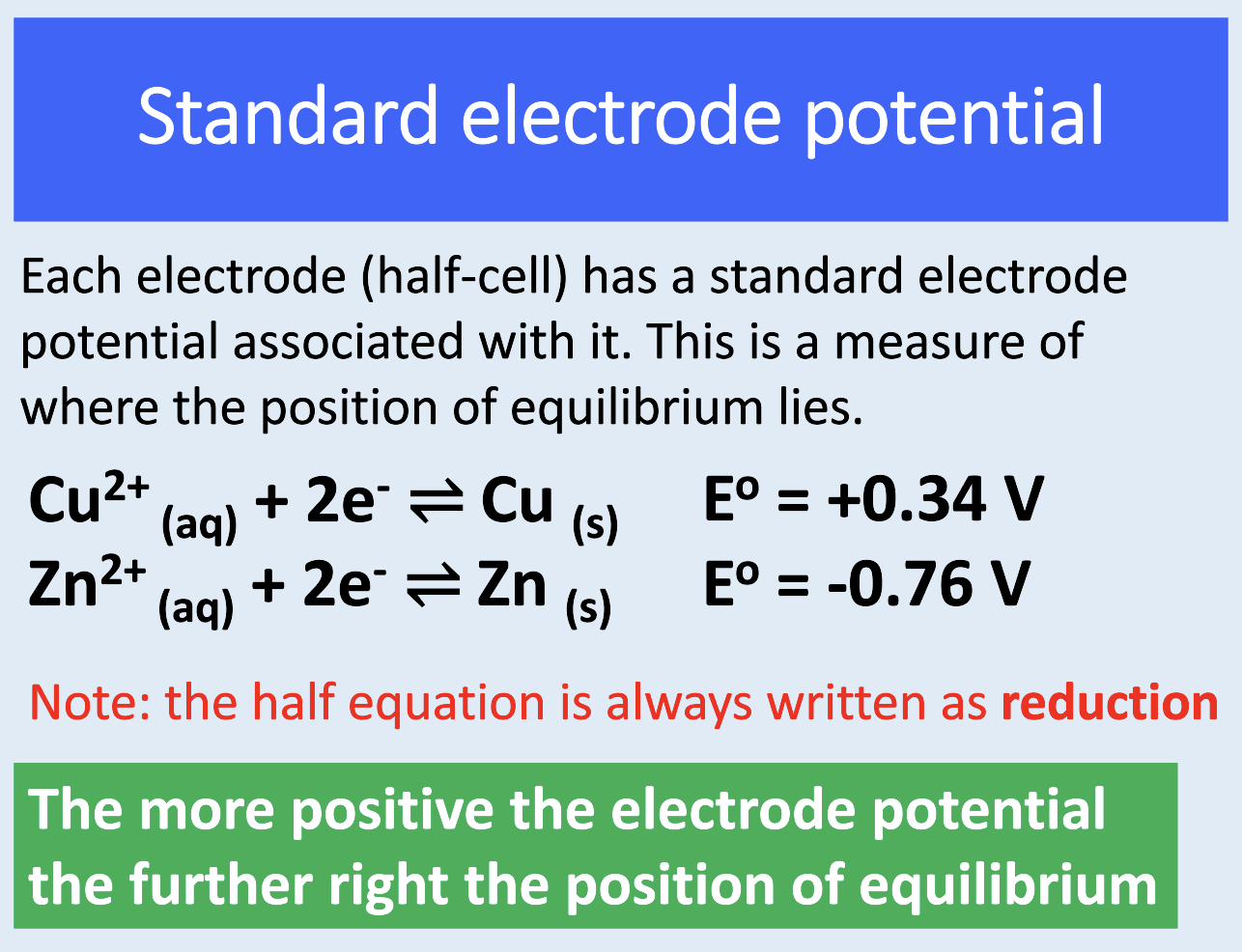
what is the standard electrode potential (Eθ)?
p.d. measured under standard conditions when the ½ cell is connected to a standard hydrogen electrode
a measure of where the PoE lies for each ½ cell
how can we relate electrode potentials to PoE?
the more +ve the electrode potential, the further right the PoE
how are ½ equations always written for standard electrode potentials?
always written as reduction (see image for e.g.)
what can we use to measure the Eθ for a single ½ cell?
standard hydrogen electrode = electrode consisting of H2 gas in contact w/ H+ ions on a platinum surface
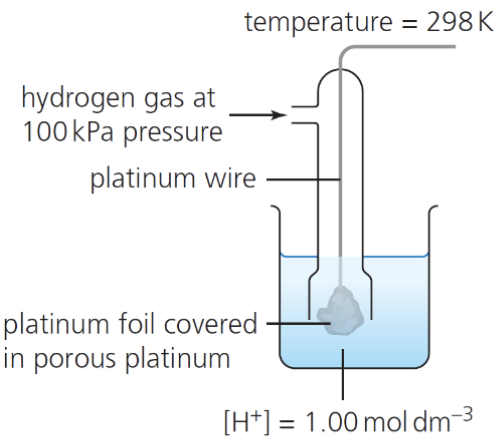
describe how we can use the standard hydrogen electrode to measure the Eθ for a single ½ cell:
H2 gas flows over the inert Pt electrode to establish the equilibrium 2H+ (aq) + 2e- ⇌ H2 (g)
the standard hydrogen electrode is connected to the ½ cell under investigation under standard conditions
the Eθ of the standard hydrogen electrode is 0.00 V by definition
so we can determine the Eθ by using the formula Ecell = Eright - Eleft
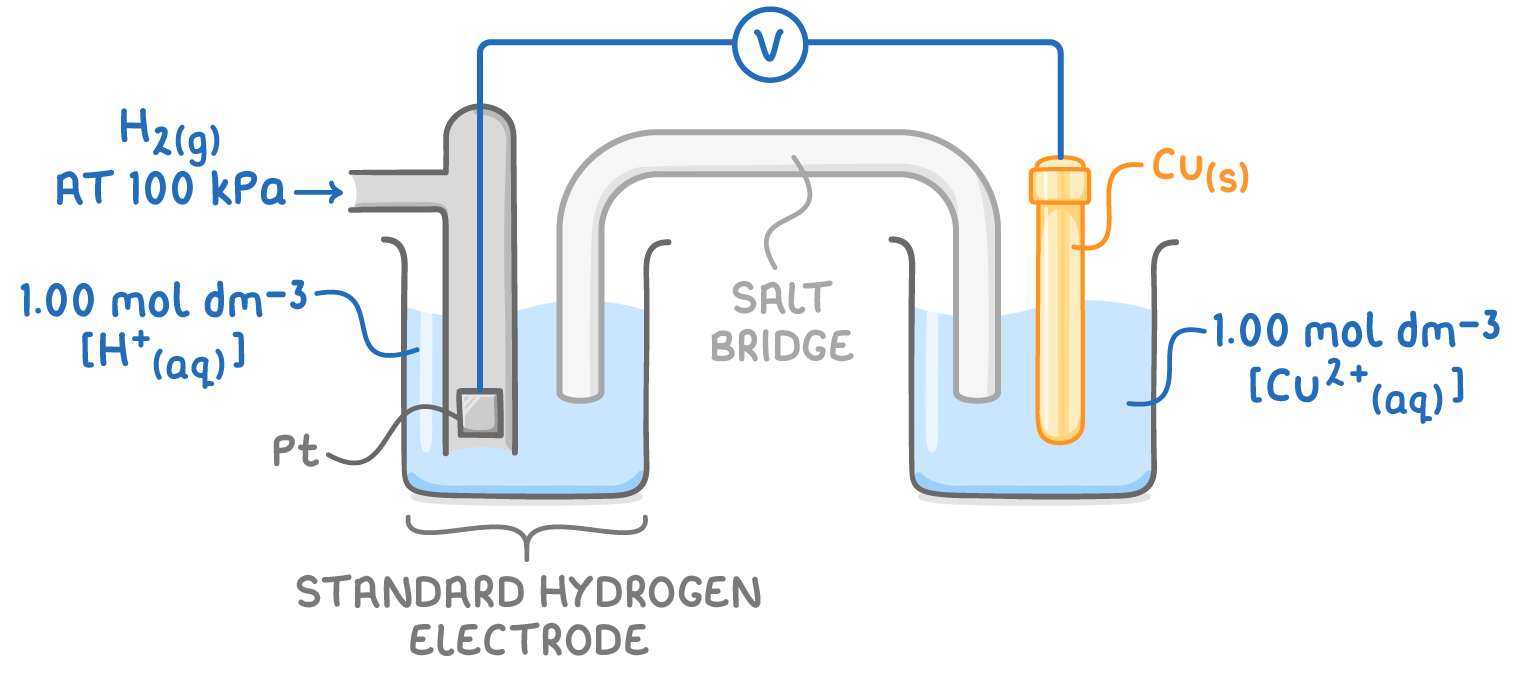
state the standard conditions necessary when using the standard hydrogen electrode:
298 K
100 kPa
all solns have a conc of 1 mol dm-3
which side is the standard hydrogen electrode always positioned on?
LHS - regardless of Eθ values of other cells
give the formula used to calculate the EMF (Ecell) of a cell:
Ecell = Eright - Eleft
give the symbols used in cell notation and state what they represent:

which species is placed closest to the salt bridge || when writing cell notation?
species w/ the highest oxidation state (i.e. oxidised species)
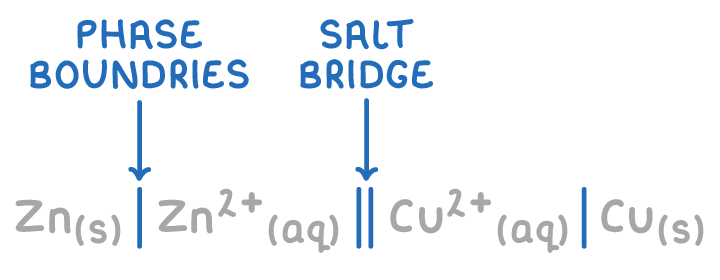
on which side of the salt bridge || do you write each species in cell notation?
as seen/given in a diagram:
-ve ½ cell (oxidised) on LHS
+ve ½ cell (reduced) on RHS
why is Pt used as the contact electrode in the standard hydrogen electrode?
inert
give and explain the cell notation for the standard hydrogen electrode being used to measure the EMF of a Cu/Cu2+ ½ cell:
Pt (s) | H2 (g) | H+ (aq) || Cu2+ (aq) | Cu (s) :
species w/ highest oxidation state closest so salt bridge
| separate species w/ diff states
Pt (s) included - used as a contact: solid contact must be used if there are only aq/gaseous species