Pharmacology
1/135
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
136 Terms
Explain the fluid mosaic model of plasma membrane
Flexible phospholipid bilayer with embedded proteins + cholesterol providing structural integrity
3 types of transport mechanisms in cell membranes
passive diffusion: no membrane proteins → down conc gradient
Facilitated diffusion: use membrane protein → down conc gradient
Active transport: against conc gradient + requires ATP
What is the most important membrane transport mechanism in the control of intracellular Na/K+ conc
Na-K ATPase - pumps 3Na out + 2K+ in for every ATP molecule
How is cell volume regulated
Na+, K+, Cl- are osmotically active ions so water will follow
IV fluid prescribing (adults)
water: 25-30ml/kg/day
Na/K/Cl: 1mmol/kg/day
Glucose: 50-100g/day
Crystalloid
Aqueous sol composed of water + electrolytes/glucose
Can be isotonic, hypo or hypertonic
Advantages of crystalloids
cheap
Non allergic
Coagulation not effected
Crystalloid disadvantages
higher vol needed
Short time in intravascular space
Examples of crystalloids
0.9% saline
Hartmann’s (closest composition to plasma)
Glucose
Ringer’s lactate
What is fluid resuscitation used for
maintains intravascular vol in hypotensive/shock
Replaces large fluid loss
How do colloids work
Large molecular weight particles cannot pass through capillary membrane → creates osmotic gradient
water remains in intravascular space (high oncotic pressure)
Colloid disadvantages
expensive
Risk of anaphylaxis
Coagulopathy
Colloid examples
albumin
Gelaspan
Dehydration vs volume loss
Dehydration = loss of total body water
Vol loss = reduced ECF
Symptoms of reduced ECF
Thirst
Muscle cramps
Nausea/vomiting
Confusion
Postural dizziness/hypotension
Clinical features of increased ECF
peripheral/pulmonary oedema
Ascites
Raised JVP
Basal crackles
What is a ligand + examples
A molecule that reversibly binds to a receptor
Exogenous: drugs
endogenous: neurotransmitters, hormones
What is the difference between a receptor and acceptor
Receptors Specialised proteins dependent on ligand binding
Acceptors can be functional independently
What are agonists
Drugs that bind to receptors + produce a response
Types of receptors
ionotropic
GPCR (metabotropic)
Enzyme-linked
Intracellular
Examples of ionotropic receptors
Cholinergic nicotinic
GABAa
5-HT3
Types of cholinergic receptors
muscarinic
Nicotinic
Muscarinic receptors
5 types:
M1,3,5 → GPCR (Gq) mediated by inositol lipid signaling
M2,4 → use Gi to open K+ channels = hyperpolarisation
Locations of muscarinic receptors
M1 - CNS+PNS parietal cells (neural receptors)
M2 - cardiac
M3- glandular + smooth muscle
M4/5 - CNS
How do nicotinic receptors work
Ligand gated ion channel
Ach binds to N terminal → conformational change
Channel opens so Na+ in/K+ out (depolerisation)
How do GCRP work (metabotropic)
Activate intracellular guanine nucleotide binding protein
Bind to GDP when inactive - GTP when active
Adrenoceptors → respond to catecholamines
Muscarinic → respond to ach
Enzyme/kinase linked receptors
hormones, growth factors, cytokines
Extracellular ligand binding site
Intracellular catalytic domain → phosphorylates substrate
What is the significance of autophosphorylation
Receptor remains active until phosphate group is removed
So able to interact with other proteins → signalling
Intracellular receptors
Typically act on DNA → eg genetic expression of: enzymes, cytokines, receptor proteins
Examples of drugs that interact with intracellular receptors
sex hormones
Thyroid hormones
Mineralcorticoids
Vitamin D
Steps of cell signalling pathway
1) reception
2) transduction
3) response
Explain receptor mediated endocytosis process (eg LDLs + transferrin)
1) ligand binds to receptor in clathrin coated pits
2) invaginates + buds off from plasma membrane → vesicle
3) vesicle fuses with endosome → separates ligand from receptor (uses ATP)
4) receptors recycled back to CSM + ligand vesicle digested by lysosome
How do local anaesthetics work
bind to voltage-gated Na+ channels (intracellular side of receptor)
No Na+ entry = no AP generated
G-protein couple receptor structure
seven-pass trasmembrane receptor
Single polypeptide chain
Extracellular N terminal + intracellular C terminal → activates G protein
G protein structure
alpha, beta and gamma subunits
3 types q,i,s
Inactive = bound to GDP, Active = bound to GTP
Examples of drugs that target GPCR
bronchodilators
Beta blockers
ARB
Antihistamines
Naloxone
What enzyme does Gq activate +function
Phoshoplipase C: cleaves PIP2 → IP3+ DAG
IP3 = opens Ca2+ channels in endoplasmic reticulum so increased conc inside cell
DAG = phosphorylates protein kinase C
What enzyme does Gs stimulate + function
Adenylyl cyclase: converts ATP → cAMP
CAMP: binds to regulatory subunit of protein kinase A → catalytic subunit phosphorylates target proteins
What enzyme does Gi effect
INHIBITS adenylyl cyclase →Gs negative feedback
Examples of Gs-coupled receptors
beta adrenoceptors
D1 dopamine
H2 histamine
Examples of Gi-coupled receptors
alpha2 adrenoceptor
D2 dopamine
Opioid
Naloxone
Short acting opioid antagonist
Used to treat respiratory distress in opioid overdose
Opioids mechanism of action
Mu, delta + kappa receptor agonists (brain, spinal cord, GI tract)
Pre-synaptic: Ca+ channels inhibited so less Ca+ = decreased neurotransmitters
Post-synaptic: K+ channels open so K+ efflux = hyperpolarisation
Opioid side effects
nausea
Constipation
Miosis (Pupil constriction)
Respiratory distress
How is Ca2+ gradient formed + maintained
PMCA + Na+/ca2+ exchanger
Ca2+ buffers
Intracellular Ca2+ stores
Closed VOCC at resting membrane potential
Plasma membrane Ca2+ ATPase (PMCA) mechanism
high intacellular [Ca2+] → calmodulin to binds to Ca2+
Ca2+-calmodulin binds to PMCA → removes Ca2+
High affinity, low capacity
How do Ca2+ buffers work
binding proteins that limit ca2+ diffusion
Decrease intracellular conc by binding to excess ca2+
Mechanism of action of sodium channel blockers as antiarrhythmic
Block Na+ channel so no influx
Reduced phase 0 slope = reduced magnitude of AP + slow rate
A- moderate, B- weak, C-strong
Examples of sodium channel blockers
Class A: disopyramide, quinidine
Class B: lidocaine, mexiletine
Class C: encainide, flecainide
Beta blocker mechanism of action
block cardiac beta1-adrenoceptors = no catecholamine binding
Decreased SANS activity +cAMP
Decrease phase 4 slope + increase PR interval
slows AV conduction = less contractility
Examples of beta blockers
selective: atenolol, bisoprolol
Non-selective: propanolol, Timolol
Potassium channel blocker mechanism of action
channel blocked = no k+ efflux
Increased phase 2 + delays phase 3
Prolongs AP → increased absolute refractory period
Examples of potassium channel blockers
amiodarone
Ibutilide
Droneradone
Calcium channel blocker mechanism of action
blocked channel = no Ca2+ influx
Decreased phase 0 + 4 slope, increased PR interval
Slows AV conduction = slower velocity + decreased contractility
Examples of calcium channel blockers
verapamil
Diltiazem
What are antagonists
molecule that blocks binding site + inhibits response → affinity but no efficacy
Can be reversible or irreversible competitive, or non-competitive
Pharmacodynamics
mechanism + effects of a medication binding to receptors on the body
What is drug potency + how is it measured
amount of drug required to elicit pharmacological effect
EC50 = drug conc producing 50% of max effect
On graph more potent drug = left (lower dose)
How is the safety of a medication measured
therapeutic index
TD50 (dose that causes toxic side effects in 50% pop)/ ED50
Narrow complex (ratio closer to 1)→ greater danger
What is drug efficacy
measures strength of drug action once bound to receptor
what is drug affinity + how is it measured
measures the tendency of a drug to bind to receptors
Dissociation constant - conc of drug at which half the receptors are occupied (low Kd = higher affinity)
What is a partial agonist
molecule that binds to receptor but elicits a weaker response (reduced efficacy)
What is an allosteric modulator
substance that binds to secondary site on receptor + changes agonist binding site (orthosteric)
Can increase or decrease affinity
How do inverse agonists differ from antagonists
IA produces the opposite effect to an agonist
antagonists block the effects of both
Define tachyphylaxis + tolerance
Tachyphylaxis: rapid decrease of drug effect following repeated administration
Tolerance: gradual decreased response to drug
Define refractoriness + resistance
Refractoriness: loss of therapeutic efficacy
Resistance: loss of efficacy of chemotherapeutic agents
What mechanisms can cause a reduced drug response
conformational change in receptor
Translocation of receptors
Increased metabolic degradation
Physiological adaptation
What is bioavailability + what can affect it
Proportion of administered drug (non-IV) that reaches systemic circulation
solubility
GI absorption
Hydrolysis by acid or enzymes
Examples of enteral drug administration
Drug entering via GIT
oral
Rectal
Buccal (between cheek)
sublingual (under tongue)
Examples of parenteral administration
Bypasses GIT directly into systemic circulation
Intravenous
Subcutaneous
Intramuscular
What is first pass metabolism of enteral drugs
blood from GIT is directed to liver
Drug metabolised before reaching systemic circulation = reduced efficacy
Define pharmacokinetics + steps (ADME)
Movement + modification of drugs within the body
Absorption
Distribution
Metabolism
Excretion
Examples of drugs affected by first pass metabolism (NIL By Mouth)
nitrates
Imipramine
Lidocaine
Beta blockers
Morphine
Nitroglycerine mechanism of action
oral/sublingual for rapid absorption + relief (angina)
Nitrite converted to NO → diffuses into SM cells of arteries + veins = vasodilation
Veins: peripheral blood pooling = decreased preload
Arteries: decreased vascular resistance = decrease afterload
Natural corticosteroid (hydrocortisone) MoA
binds to glucocorticoid receptors
Inhibits phospholipase A2, NF-kappa B, inflammatory transcription factors
Decreased vasodilation + capillary permeability → anti-inflammatory
Reasons to use parenteral drug administration
drug poorly absorbed by GIT
Significant first-pass metabolism
GIT not in use
Common sites of IM injections
deltoid
Rectus femoris
Gluteus medius
IM muscle contraindications
allergy
Myopathy
Muscular atrophy
Infection at administration site
Haloperidol mechanism of action
antipsychotic used for schizophrenia
D2 antagonist → competitively blocks post-synaptic receptors
IM administration = higher bioavailability, oral = maintenance
Intrathecal administration
Drug injected into spinal canal - eg spinal anaesthetic
Fine needle injects local into subarachnoid space
Delivered directly to CSF
How does an epidural differ from intrathecal administration
Epidural diffuses from dura to CSF
Given cervical, thoracic or lumbar → Catheter placed in epidural space
Examples of narrow therapeutic index (NIT) drugs
digoxin
Warfarin
Lithium
Theophylline
Levothyroxine
Therapeutic drug monitoring (TDM) + significance
measuring drug conc in blood at timed intervals to maintain a constant conc in circulation
Important to achieve therapeutic effect + avoid toxicity
Direct oral anticoagulant (DOAC) mechanism of action
Direct thrombin or factor Xa inhibitors
Prevents thrombus formation
Eg apixaban
What are the advantages of DOACs to warfarin
warfarin has narrow therapeutic index so requires INR monitoring
Pharmacokinetics: Absorption
passage of a drug from site of administration into the plasma
What are the factors affecting drug absorption?
food (enhance or impair)
Formulation
Route of administration
Pharmacokinetics: Distribution
Movement of a drug from circulation to body tissue + relative proportions of drug in the tissue
What are the factors affecting drug distribution?
plasma protein binding competition
Drug receptor sites
Regional blood flow + vascularity (brain, liver, kidney)
Lipid solubility (cross membranes easier)
Plasma protein binding
drugs will bind to plasma proteins eg albumin → limited to plasma so essentially inactive
Unbound drugs are free to act at receptor site + diffuse into tissue
What are the factors which can increase the fraction of unbound drug?
renal impairment → high blood urea + plasma proteins can be filtered out
Low plasma albumin levels → chronic liver disease, malnutrition
Late pregnancy → decreased albumin production + increased blood volume= dilution
Volume of distribution (Vd)
theoretical vol that accommodates all the drug distributed in the body
Vd = dose administered (mg)/ plasma concentration of drug (mg/L)
High vs Low volume of distribution
high Vd = higher dose needed to reach target plasma conc, as more distribution to tissue
Low Vd = lower dose required, tends to remain in plasma
Object + precipitant drug
object: used at dose lower than no. Albumin binding sites (eg warfarin)
Precipitant: used at doses greater than no. Of binding sites so displace object drug (eg aspirin)
Pharmacokinetics: Metabolism
biochemical modification of drug into +/- active metabolite - primarily in liver
phase 1: Oxidation, reduction and hydrolysis via CYP450, form more soluble + reactive products
Phase 2: Conjugation, usually form inactive + readily excretable products
What are the factors affecting drug metabolism?
first pass metabolism
Hepatic blood flow
Age
Genetics
Liver disease
Other drugs → hepatic enzyme inhibitors/inducers
Loading dose
initial higher dose given at start of treatment (single bolus)
Eventually drops down to lower maintenance dose
Useful for drugs with long half life when rapid effect desired
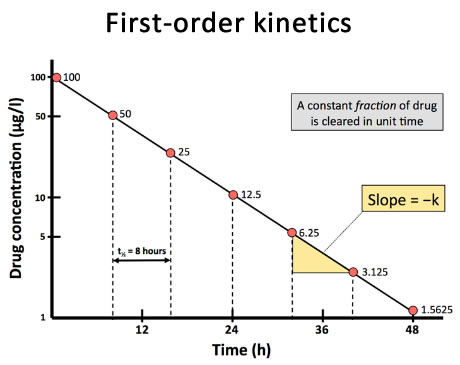
Half life
time required for drug conc in plasma to be reduced by 50%
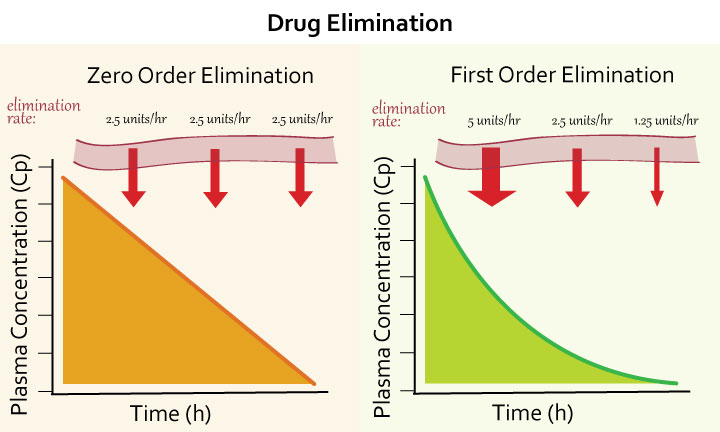
Zero order kinetics
Constant rate of drug elimination per unit of time
Eg warfarin, aspirin
First order kinetics
Rate of drug elimination is directly proportional to plasma concentration Common in most drugs
Exponential decrease → helps to determine half life