Lab 7: Transfer of Hydrogenation of Olive Oil Lab
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
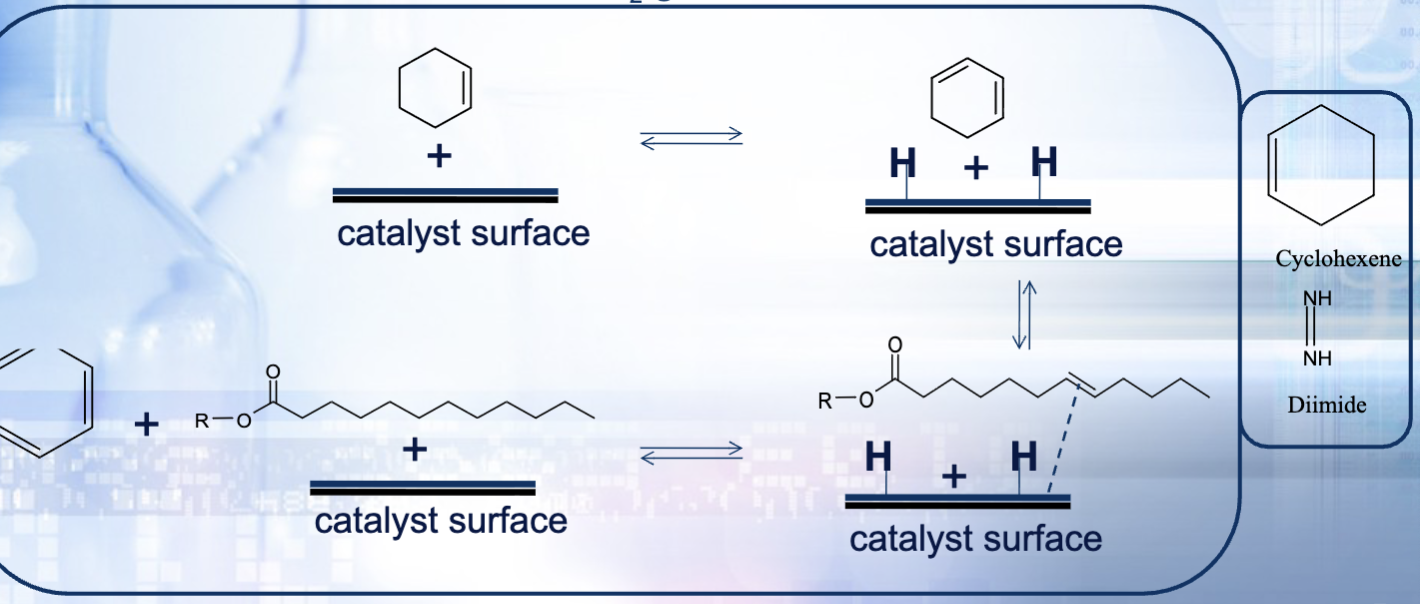
What is the general reaction scheme? (what is the hydrogen donor, the driving force for each step—there are two) *You do not need to know this!
Step 1: cyclohexane is the hydrogen donor and the driving force is the formation of benzene (highly stable aromatic ring)
We really only focus on the formation of benzene but here is step 2
Step 2: diimide (NH=NH) is used as the hydrogen donor and the driving force of the reaction is the formation of N2 gas
Balanced equation
See photo
Why are we using cyclohexane instead of regular H2 gas?
We use cyclohexane because it is compound that transfer molecular H2, so this a convenient and safe alternative method instead of using flammable/explosive H2 gas
What is the driving force of this reaction (aka why does it go to completion?)
The formation of benzene (because it is really stable)
How can you know that it went to completion?
If it properly soliidifies
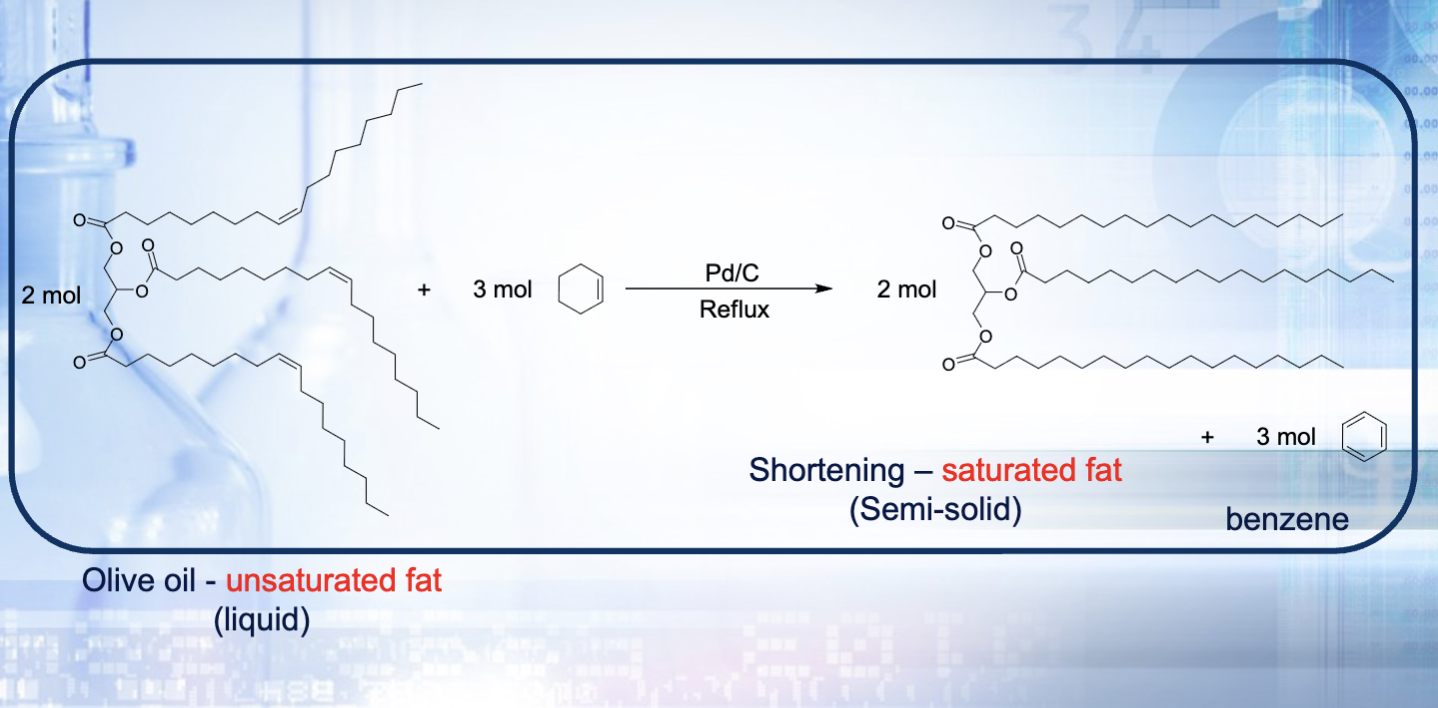
Tristearin (the product) is solid at room temperatures, so when we put it in ice bath, solidification means our reaction went to completion (reminder olive oil is a liquid at room temperature)
OR melting point determination (compare to literature value)
Why are using Pd/C? What is its role? Would the reaction proceed faster without it?
We use Pd/C because it provides a metal surface that binds H2 to the alkene, which is why the H atoms are added syn across the pi bond. The reaction would not go faster without this catalyst. The surface is needed for the reaction to occur in the first place (basically we need the metal surface to connect the H atoms and the alkene)
What are saturated and trans fats? How is hydrogenation related to this? Structure wise, how are saturated fats different from trans fats?
Saturated fats are unhealthy and raise blood cholesterol levels and clog arteries
Trans fats help convert liquid oils to solid fats because of the carbon carbon double bond
Hydrogenation of trans fats→saturated fats (removing that carbon carbon double bond)
What is hydrogenation?
The addition of hydrogen to an alkene, decreasing the number of unsaturated carbon carbon double bonds while increasing the number of saturated bonds (aka removing the C-C double bonds)
What does hydrogenation require?
A suitable catalyst and high temperatures and high pressure
Oxidation vs reduction
Oxidation: increase the number of bonds to more electronegative elements and/or reduce the number of bonds to hydrogen
Reduction: increase the number of bonds to hydrogen and/or decrease the number of bonds to electronegative elements
Hydrogenation reaction conditions (2 parts)
catalyst
hydrogen gas or alternate source of H2
Describe the metal catalysts most commonly used for hydrogenation?
Transition metal catalysts (ex: platinum, palladium, rhodium, ruthenium. and nickel based)
ex: palladium on carbon (Pd/C), platinum oxide, raney nickel
Experimental setup
using microscale glassware for the experiment (mini condenser)
Low flow of water for condenser
Filter pipette with glasswool and celite powder
General procedure
get your olive oil in a conical vial
Add palladium on carbon to the vial and then cyclohexane (DO NOT MIX)
Add a spin vane
Set up reflux with microscale condenser
Reflux for 45 minutes
Assemble celite pipet filtration apparatus: insert small ball of wool or cotton and then add 1 inch of celite
Pour 1 mL of hexane to compact the celite in the pipet
Pour your reaction mixture (make sure that all the palladium particles get caught in your celite)
Place your filtrate (the liquid that come out of the pipet) on a hot plate to remove filtration solvent and liquid byproduct (benzene)
this is judgment based: evaporate until you think it is done (little should be left and it should be viscous)
Put sample in ice water to help with solidification of the hydrogenated olive oil (tristearin)
Determine melting point range using warm water bath (slowly warm the water bath until your fat melts; record temperature of water)
Perform IR
What were the chemicals used in this experiment?
hexane
triolein (olive oil)
tristearin (hydrogenated olive oil)
Cycleohexane
hexane
benzene
10% Palladium on carbon
Experimental techniques used
Reflux, gravity filtration, IR spec
What glassware is used
Beaker, condenser, thermometer
Limiting reagent
Olive oil
What math did you do?
Limiting reagent, theoretical yield, percent yield
Compare and contrast the melting point of your final product with the literature value
The experimental value is broader and deeper than the literature value. This is because impurities, like palladium, can lower the melting point of our product. This is because the impuritieis inhibit the formation of the solid’s crystal lattice structure, weakening the intermolecular forces→ requires less energy to melt the product
How could you improve the methodology of your lab?
we could have spent more time boiling off our benzene and filtration solvent. We had a higher percent yield, but we think this is because we had some of our byproduct and solvent still present
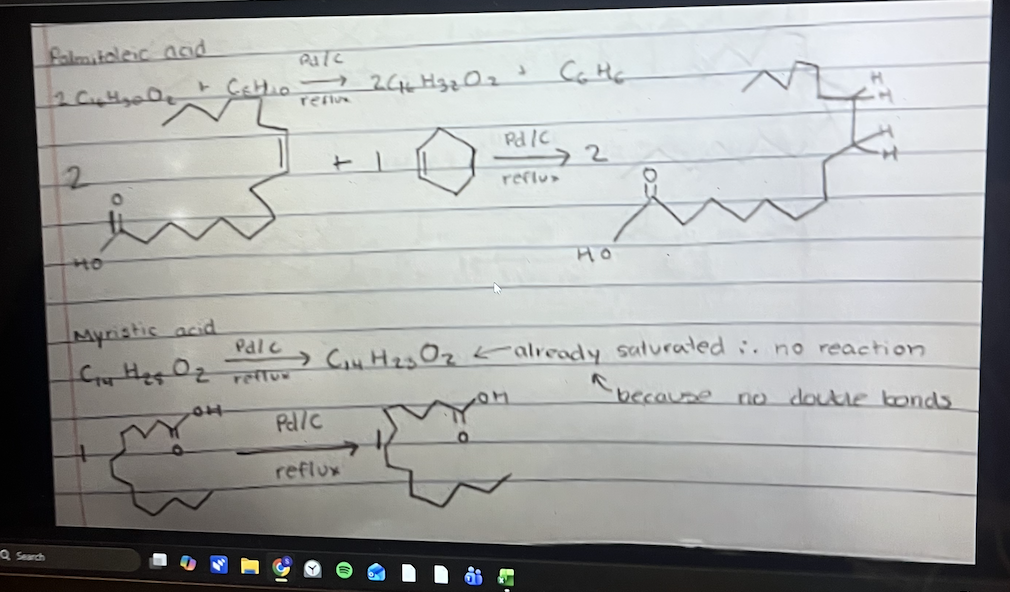
Provide the balanced equation of palmitoleic + benzene → myristic acid AND stearidonic acid + benzene → erucic acid
See photo
Notice how the number of double bonds disappear
Liquid bromine is a dark maroon/brown color. 1,2-dibromo alkanes, on the other hand, are colorless. What would it mean if you added liquid bromine to your final transfer hydrogenation product and the resulting mixture converted from dark maroon to clear upon heating? Explain your reasoning:
Bromine is needed to break double bonds (alkenes), so if you add it to your final product and there was a color change, your reaction did not go to completion
color change=there are still alkenes present (which should not happen if your reaction goes to completion)
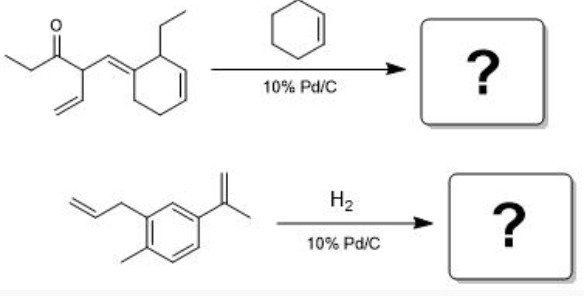
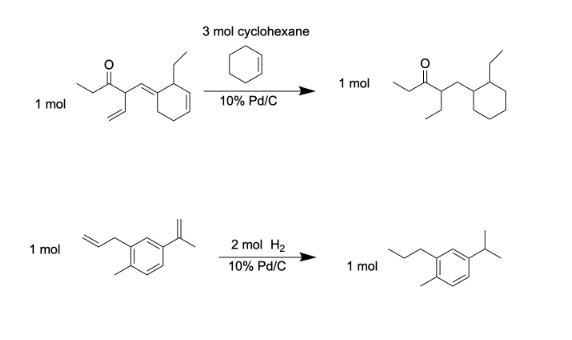
Predict the product(s) and provide a fully balanced chemical equation for each of the following hydrogenation reactions.
See photo (you need one mole of cyclohexane for each double bond broken (you do not break the double bonds in carboxyl groups and arenes when for hydrogenation)