AQA GCSE Single Science Chemistry: Bonding, structure, and the properties of matter
1/29
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
30 Terms
What is ionic bonding?
This involves particles with oppositely charged ions
Compounds are formed from metals and non-metals
Explain the electron transfer in ionic bonding
Electrons in the outer shell of the metal are transferred to the non-metal
Metal atoms lose electrons to become positively charged ions
Non-metal atoms gain electrons to become negatively charged ions
This can be represented by a dot and cross diagram
What is an ionic compound?
An ionic compound is a giant structure of ions.
These are held together by strong electrostatic forces of attraction between oppositely charged ions.
These forces act in all directions in the lattice and this is called ionic bonding.
What are the properties of ionic compounds?
They have high melting and boiling points because of the large amounts of energy needed to break the strong bonds
They can conduct electricity when melted or dissolved in water as the ions are free to move and so charge can flow
What are the positives and limitations of dot and cross diagrams?
It shows how ionic bonds are formed
It also shows the ratio in which the atoms react
However, it does not show how the ions are arranged in space
What are the limitations of ball and stick diagrams?
It is not to scale
It gives no information about the forces of attraction between the ions, or the movement of electrons to form the ions
What are the positives and limitations of 2D diagrams?
It is the easiest model to draw
However, it does not show where the ions are located on the other layers.
What are the limitations of 3D diagrams?
It is not to scale
It gives no information about the forces of attraction between the ions, or the movement of electrons to form the ions
What is covalent bonding?
This involves particles that are atoms which share pairs of electrons
It occurs in most non-metallic elements and in the compounds of non-metals
Explain the electron transfer in covalent bonding
When atoms share pairs of electrons, they form covalent bonds
The bond between atoms are strong
What might a covalently bonded substance have?
Very large molecules, such as polymers.
Giant covalent structures, such as diamond and silicon dioxide.
What is metallic bonding?
This consists of giant structures of atoms arranged in a regular pattern.
The electrons from the outer shells of the metal atoms are delocalised, and are free to move through the whole structure. This sharing of delocalised electrons results in strong metallic bonding.
This occurs in metallic elements and alloys
What are the properties of metals and alloys?
High melting and boiling points as they have giant structures with strong metallic bonding
Metals can be bent and shaped as atoms are arranged in layers
Pure metals are too soft for use so are mixed with other metals to make hard alloys
Why are alloys harder than pure metals?
Alloys contain differently sized atoms
This distorts the regular arrangement of atoms
Layers of atoms can no longer slide over another
Why are metals good conductors?
They are good electrical conductors because delocalised electrons in the metal carry electrical charge through the metal
They are good thermal conductors because energy is transferred by delocalised electrons
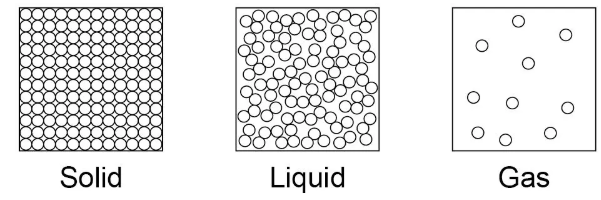
What are the limitations of this simple model?
In the model there are no forces
All particles are represented as spheres
The spheres are solid.
What are the properties of simple molecules?
They have relatively low melting and boiling points as they have weak intermolecular forces that are easily overcome
These forces increase with the size of the molecules so larger molecules have higher melting and boiling points
They do not conduct electricity as the molecules do not have an overall electric charge
What are polymers?
These have very large molecules
Atoms are linked to other atoms by strong covalent bonds
Intermolecular forces are relatively strong
These substances are solid at room temperature
What is the structure and properties of diamond?
Each carbon atom forms four covalent bonds with other carbon atoms in a giant covalent structure
It is very hard
It has a very high melting point
It does not conduct electricity
What is the structure and properties of graphite?
Each carbon atom forms three covalent bonds with three other carbon atoms
There are layers of hexagonal rings which have no covalent bonds between the layers.
There are weak forces of attraction between the layers- meaning graphite is soft
Similarly to metals, one electron from each carbon atom is delocalised- meaning graphite has good electrical conductivity
It has many strong covalent bonds- which mean graphite has a high melting and boiling point
What is the structure and properties of graphene?
This is a one-atom-thick, single layer of graphene and so has the same structure
This is very strong and has a low density
It can conduct electricity
What are the uses of graphite and graphene?
Graphite is used as a lubricant and in pencils
Graphene is used in solar cells and batteries, as well as targeted drug delivery
What are fullerenes?
These are molecules of carbon atoms with hollow shapes.
The structure of fullerenes is based on hexagonal rings of carbon atoms but they may also contain rings with five or seven carbon atoms.
The first fullerene to be discovered was Buckminsterfullerene (C60 ) which has a spherical shape, has a low melting point and is slippery
What are carbon nanotubes?
These are cylindrical fullerenes with very high length to diameter ratios
They have high tensile strength and are good electrical conductors
Their properties make them useful for nanotechnology, electronics and materials.
What are the uses of fullerenes?
Fullerenes are being used within the field of medical science.
This includes being used as light-activated antimicrobial agents.
It is also used in medical imagery as contrast agents in MRIs and x-rays.
What are the applications of nanoparticles?
In medicine
In electronics
In cosmetics
Suncreams
As deodorants
As catalysts
What are the two main advantages of nanoparticles?
The tiny size of nanoparticles compared to the same material in bulk
The large surface area to volume ratios of nanoparticulate materials compared to the same material in bulk
What are the risks of nanoparticles?
Some people are concerned that the small size of nanoparticles makes it possible to breathe them in, or for them to pass into cells.
Once inside the body, they might catalyse reactions that are harmful.
Toxic substances could bind to them because of their large surface area to volume ratios, harming health if the nanoparticles do get into the body.
Modern nanoparticulate materials have only become common recently, so it is difficult for scientists to determine their risks.
List the sizes of nanoparticles, fine particles and coarse particles
Nanoparticles: 1 x 10-9 m – 2.5 x 10-7 m
Fine particles: 1 x 10-7 m – 2.5 x 10-6 m
Coarse particles: 1 x 10-5 m – 2.5 x 10-6 m
What is the relationship between the length of a cube and its surface area?
As the side of cube decreases by a factor of 10 the surface area to volume ratio increases by a factor of 10.