mod 2 slide 2 (solid organ transplantation & HSCT)
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
what are the 3 components of organ transplantation?
organ, tissue, or cell
transplant (nonself, self)
rejection
solid organ transplantation (SOT)
new advancements in surgical techniques have allowed for more efficient and refined multi-organ procurements with minimal complications and decreased ____ ____ ____
immunosuppression therapy has also seen advancements with the expansion of immunosuppressive protocols to dampen the host immune response and improve _____ and ____ ___ ____ ____
the field of SOT faces new barriers, most importantly the expanding demand for SOT that is outpacing the current supply
______ protocols have been developed in an attempt to address these concerns
ischemic injury events
short and long-term graft survival
allocation
solid organ transplantation
the transplant of an organ or tissue from one individual to another (graft) of the same species with a different genotype is called a(n) _____
the transplant between individuals of different species is called a(n) ______
where do allografts come from?
allograft
xenograft
cadavers, living-related, and living-unrelated donors
organ transplantation and rejection
kidney transplantation is the optimal treatment for patients with what? — who would otherwise require dialysis
people who require a new kidney have ______ and/or _____ as a cause of chronic renal failure and ESRD
patients with ESRD are at dramatically increased ______ risk compared with the general population
ESRD
HTN and/or diabetes
CVD
organ transplantation and rejection
worldwide, CVD remains the leading cause of what?
what are also major causes?
the new kidney is transplanted into a _________ neighborhood
transplanting an organ/tissue from donor or recipient results in immune system responding in the normal fashion… attempts to _____ _____
death with a functioning graft (DWFG)
infection and cancer
pro-atherosclerotic
destroy non-self
organ transplantation and rejection
solution: _____ the immune system — this must be done for a lifetime
many immunosuppressive agents increase risk for ____
____ and _____ are 2 major barriers to successful organ transplantation
suppress
CVD
infection and rejection
MHC = HLA — huan leukocyte antigen
____ represents a gene product that allows T cells to interact with cells presenting antigens (APCs)
MHC is the most ______ system in the body, which makes it very unlikely that 2 individuals will express identical sets of MHC molecules (graft/transplant rejection)
what type of cells have MHC?
what is it also referred to as?
> 41,000 HLA alleles
most polymorphic are the -A, -B, -C (class I) and -DP, -DQ, and -DR (class II)
these 6 HLA alleles commonly used for what in transplantation?
on what chromosome?
MHC
polymorphic
all nucleated cells
HLA (human leukocyte antigen)
matching
human chromosome 6
haplotypes and HLA
each set of HLAs is referred to as a ____ — where do we get these?
due to the inheritance patterns, it is possible to determine the probability of sharing HLA types with a ____
you have a 25% chance of being HLA identical, a 25% chance of being completely non-identical, and a 50% chance of inheriting ½ haplotype with your siblings
odds go ____ exponentially with everyone else in the world
HLA matching generally uses HLA-___, -___, and -___ alleles (6 match system)
some agencies use 10 match system — ore difficult
haplotypes — one form each parent
sibling
down
A, B, DR
what is a balancing act between capturing the benefits of a well-matched transplant and diminishing the problems associated with achieving that transplant
transplantation
C34+
what is used to treat patients with hematological malignancies, such as non-hodgkin’s lymphoma and multiple myeloma, and to reconstitute hematopoiesis following high-dose chemotherapy — usually autologous (stem cells form own patient) or allogenic (donor)
what is the ultimate goal?
although a small number of hematopoietic cells circulate the peripheral blood at all times, _____ is necessary to drive sufficient numbers of hematopoietic stem cells from the bone marrow to peripheral circulation, where the can be harvested (collected) by ______
what eradicated the cancerous patient immune cells using a combination of high-dose radiotherapy and immunosuppressive drugs such as cyclophosphomide after stem cells have been harvested?
hematopoietic stem cell transplantation (HSCT)
to repopulate the bone marrow with a complete lineage of hematopoietic stem cells with minimal burden to the patient
mobilization; apheresis
myeloablative conditioning
hematopoietic stem cell transplantation (HSCT)
what are the steps in order?
injections
mobilization
collection
storage
freezing
chemotherapy and/or radiation
stem cell transplant
engraftment and recovery
name the drug: first in class antagonist of the CXCR4 receptor — it blocks binding of SDF1a, thereby mobilizing CD34+ cells to peripheral blood
plerixafor (mozobil)
plerixafor (mozobil)
indicated for use in combination with _____ to mobilize hematopoietic stem cells to the peripheral blood for collection
CD34 is a cell surface protein cluster of differentiation found on surface of ____ ___ — it’s an adhesion molecule which keeps stem cells attached to bone marrow — one of many cell markers
G-CSF
stem cells
transplantation: IL-2 is the key
the most important mediator of the immune response are what?
activation of these is accomplished largely by the cytokine interleukin ____
what follows a sequence of events that involves detection of donor histocompatibility differences by the recipient’s immune system, recruitment of activated T cells, activation of immune effector mechanisms, and ultimately, leading to this?
rejections are categorized as what?
T cells
IL-2
graft rejection
hyperacute, acute, chronic
occurs within minutes to days of transplantation, is due to performed IgG antibodies in the recipient that react against antigens in the transplanted organ — B cell issue
hyperacute
occurs most frequently in the first 6 mos after transplantation and is mainly mediated by T cells, which infiltrate the allograft and cause tissue destruction — immunosuppressive induction therapy is mot effective in preventing this type of rejection
acute
graft function slow deteriorates and there is histologic evidence of fibrosis — for all organs, the pathophysiology of this rejection is similar: progressive hypertrophy of the arteries and fibrosis and eventual failure of the organ transplant — immunosuppressive maintenance therapy is effective → lifetime
chronic
induction vs. maintenance therapy
what is the goal of induction therapy?
achieve immediate, profound immunosuppression for approx. 2 wks post-transplant to reduce the likelihood of hyperacute and acute rejection
induction vs. maintenance therapy
what is the goal of maintenance therapy?
reduce the immune system’s ability to recognize and reject the foreign organ or tissue, while limiting toxicity — as the patient progresses further post-transplant the risk of rejection is reduced and the immunosuppressive regimen is tailored to the individual patient to provide lifelong suppression of the immune system with minimal toxicity
what is the best maintenance immunosuppressive regimen?
although an adequate level of immunosuppression is required to dampen the immune response to the allograft, the level of chronic immunosuppression is slowly decreased over time (as the risk of acute rejection decreases) to help lower the overall risk of ____ and _____ — these risks directly correlate with the degree of overall immunosuppression
the type of immunosuppression may also be varied to decrease the risk of developing _____ _____ ____, the most common underlying long-term cause of allograft loss
immunosuppressive regimens vary among transplantation centers — selection is _____ specific
what is required for all patients?
infection and malignancy
chronic allograft nephropathy (CAN)
patient
therapeutic drug monitoring
induction agents
primary reason for the use of induction therapy, or intense immunosuppressive therapy at the time of transplant, is to avoid what?
2 classes — what are they?
acute rejection
T cell depleting agents
IL2 receptor blockers
thymoglobulin
is a purified ____ ____ ____ obtained by immunization of rabbits with human thymocytes (T cells)
is indicated for the treatment of what in conjunction with immunosuppresion?
in patients, profound T cell depletion is usually observed within how many days from initiating thymoglobulin therapy?
should be used under ____ medical supervision in a hospital setting, and patients should be carefully monitored during the infusion
most commonly used induction agent in the US
IgG based polyclonals
renal transplant acute rejections
2 days
strict
basiliximab (simulect)
binds specifically to IL2 receptor on surface of activated T cells — it’s a(n) _____ ____ ____
competitively inhibits IL2 activation of T cells — what is it a critical pathway in?
indicated for induction therapy in patients receiving what kind of transplants? — used in conjunction with immunosuppressants
IV admin
not considered as potent an immunosuppressive agent as thymoglobulin but has a much more _____ adverse-effect profile and is most commonly used in patients at ____ risk for acute rejection
IL2 receptor antagonist (IL2 RA)
the cellular immune response in transplant rejection
kidney
favorable; low
maintenance agents
maintenance immunosuppressive therapy is administered to who?
why?
adverse event profiles vary among drugs
all transplant recipients
to help prevent acute-chronic rejection and the loss of the renal allograft
mTOR inhibitors (mTORi): sirolimus (rapamune) and everolimus (zortress)
small molecules, oral admin
clinical trials have demonstrated that everolimus, in combination with reduced-dose calcineurin inhibitors (CNI) like cyclosporine A or tacrolimus, is effective in preventing what?
the combined use of sirolimus and CNIs should be _____, because these agents potentiate nephrotoxicity, particularly when used in the early post-transplant period
______ has improved solubility, 60% greater bioavailability, a shorter half-life (28 vs 60 hours), and more rapid achievement of steady-state levels (4 vs 6 days) than sirolimus
both metabolized by what? DDIs?
what do they both require?
when used as _____ maintenance, everolimus has been used in reduced dosages in different regimens in combination with CNIs
rejection episodes and graft loss
avoided
everolimus
CYP450 3A4 — yes, DDIs
therapeutic drug monitoring — narrow TI
long term
calcineurin inhibitors (CNI)
CNI suppress the immune system by doing what?
long term CNI use may induce _____ _____, resulting in progressive graft dysfunction
CNIs can also promote what? — which are the leading causes of premature death with a functioning graft
preventing IL2 production in T cells
irreversible nephrotoxicity
cardiovascular events, infections, and malignancies
cyclosporine
hirsutism
gingival hyperplasia
hypercholesterolemia
tacrolimus
alopecia
neurotoxicity
pancreatic islet cell toxicity leading to glucose intolerance
GI disturbances
both cyclosporine and tacrolimus
nephrotoxicity
HTN
gout
hyperkalemia
hypomagnesemia
tacrolimus (prograf)
the calcineurin inhibitor prograf is contraindicated with ____ ____
should limit the amount of time spent in what and avoid what?
are tacrolimus extended release tablets the same as prograf capsules or granules?
can they be substituted for each other?
tacrolimus dosed in mg/kg body weight/day and based on adult/pediatric patient and type of organ transplanted.
live vaccines
sunlight and avoid exposure to UV light (tanning machines)
no
no
tacrolimus (prograf) - box wanring
what is there increased risk of?
malignancies and serious infections
mycopnenolate mofiil (MMF)
prodrug that gets converted by _____ to mycophenolic acid (MPA)
what type of drug is this?
MPA is inhibitor of inosine monophosphate dehydrogenase (IMPDH) — what does it inhibit?
what is it indicated for?
box warning?
esterases
anti-proliferative drug
de novo pathway of guanosine nucleotide synthesis
prophylaxis of organ rejection (allogenic renal, cardiac or hepatic transplants)
embryofetal toxicity, malignancies, serious infections
belatacept (nulojix)
a ____ ____ composed of the Fc fragment of a human IgG linked to the extracellular domain of CTLA-4 (decoy receptor) which is a molecule crucial for T cell co stimulation
it’s a T cell co stimulation blocker that interferes with what?
indicated for prophylaxis of organ rejection in adult patients receiving a _____ transplant
what is there increased risk of developing (predominantly involving the CNS)
recipients without immunity to epstein barr virus (EBV) are at a particularly increased risk; use in EBV ______ patients only
do not use nulojix in transplant recipients who are EBV ____ or with unknown status
fusion protein
APC CD 80/86 interactions
kidney
post transplant lymphoproliferative disorder (PTLD)
seropositive
seronegative
induction and maintenance data
in the US, approx 85% of transplant recipients were discharged on ______ and _____ either with (58%) or without (42%) steriods
tacrolimus and MMF
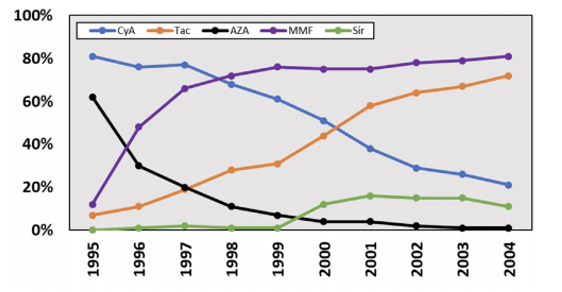
trends in immunosuppression
what does the picture show?
maintenance
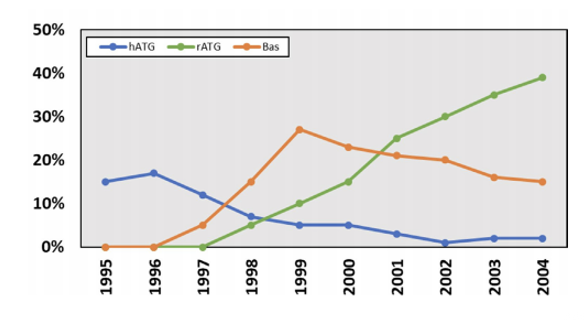
trends in immunosuppression
what does the picture show?
induction
deeper dive into tacrolimus
most common regimen combines tacrolimus with _____ (with or without steroids)
the principle challenge in immunosuppression is achieving a balance between under-immunosuppression and over-immunosuppression
the task of optimizing tacrolimus dosing in immunosuppression therapy extends beyond mere monitoring of drug levels; requires consideration of a multitude of clinical factors, including patient age, cardiovascular health, infection status, renal function, DDIs, interpatient variability, and recent graft injections
MMF
what can under-immunosuppression lead to?
graft rejections
what can over-immunosuppression lead to?
off-target toxicities and infection
deeper dive into tacrolimus
clinical use of TAC is associated with the risk of what?
has a _____ TI and is metabolized by CYP3A4 (DDIs)
tacrolimus trough level targets a range from what?
2019 — range from what?
nephrotoxicity, neurotoxicity, HTN, dyslipidemia, and posttransplant DM
narrow
5 - 20
7 - 12
tacrolimus trough
maintaining tacrolimus levels within 5 - 7.9 for the first yr and 5 - 6.9 for years 2 - 6 correlates with what?
high graft survival and optimal safety outcomes
ischemia/reperfusion (I/R) injury
harvested organs (stored on ice) are subjected to injury during cold preservation due to a _____ and _____ condition
further damage is induced at ______ when warm oxygenated blood is reintroduced into the graft
the lack of oxygen during cold preservation induces depletion of ATP and shifts to _____ metabolism by glycolysis pathway, followed by deterioration and activation of cytotoxic enzymes
subsequent warm reperfusion of grafts causes what promoting further cell damage?
hypoxic and hypothermic
reperfusion (warm reperfusion)
anaerobic
excess of oxygen and generates reactive oxygen species
ischemia/reperfusion (I/R) injury
as a result, damage or loss of vascular endothelial cells (VECs), disturbance of microcirculation, activation of potent inflammatory mediators, and inflammatory infiltration are known to be _____ features associated with I/R injuries
initially, I/R injury contributes to ____ ____ ____ — the need for dialysis within one week after renal rtansplantation
characteristic
delayed graft function (DGF)
carbon monoxide (CO)
what does it reduce?
how does it do this?
I/R injury in organ transplantation
protecting vascular endothelial cells (VEC)
acting as an anti-coagulation factor
exhibiting anti-inflammatory effects
inhibiting apoptosis
carbon monoxide (CO)
CO and allograft rejection
what does it prevent?
T cell proliferation
allograft rejection
fibrosis
what is the landmark transplant?
xenograft
schematic of MOAs of immunosuppressive drugs
T cell proliferation results from activation after presentation of ____ ____ by antigen presenting cells in conjunction with the MHC class II and B7 co-stimulation complex
this mechanism results in activation of _____
which leads to production of ____
autocrine stimulation by IL2 results in cell proliferation by a pathway involving _____ and ____ ____ ____
donor antigen
calcineurin
IL2
m-TOR and cyclin-dependent kinase
schematic of MOAs of immunosuppressive drugs
immunosuppressive agents exert their effects at a number of different targets to prevent _____ _____
drugs act at all phases of T cell activation processes
always used in combination for what?
T cell proliferation
optimal therapeutic outcomes
carbon monoxide releasing molecules (CORMS) for IRI
small molecule “____“ of CO
ruthenium carbonyl complexes
boron carbonyl complexes
water soluble, delivery of exact amounts to tissues
useful in studying the effects of CO in cells/tissues in a therapeutically relevant setting
prodrug