Valence Electrons & How To Find Them
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
Valence Electrons
Are the outermost, high-energy electrons responsible for chemical bonding and determining an element’s reactivity.
Core electrons
Electrons closest to the nucleus that are tightly bound. (the rest of electrons that are not valence electrons)
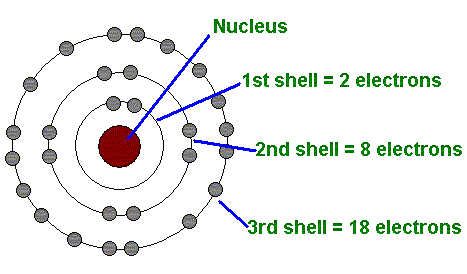
The First Energy Level (Shell)
Can only hold up to 2 electrons (see image)
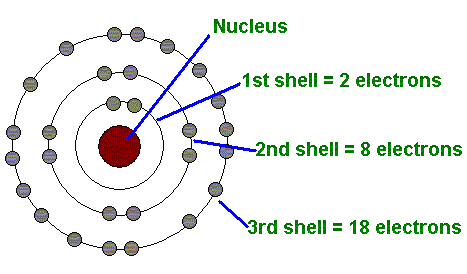
The Second Energy Level (shell)
Can have up to 8 electrons (see image)
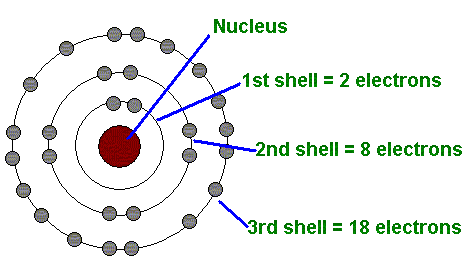
The Third Energy Level (shell)
Can hold up 18 electrons (see image)
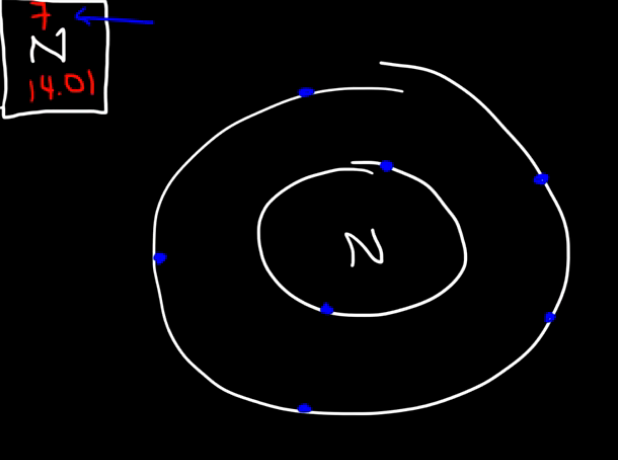
EX: Nitrogen
Nitrogen has 7 electrons. The first energy level can hold up to 2 electrons, and the second up to 8, but we only need 5 to make 7. So, Nitrogen has 5 valence electrons (the electrons in the outermost shell), and 2 core electrons. (see image)
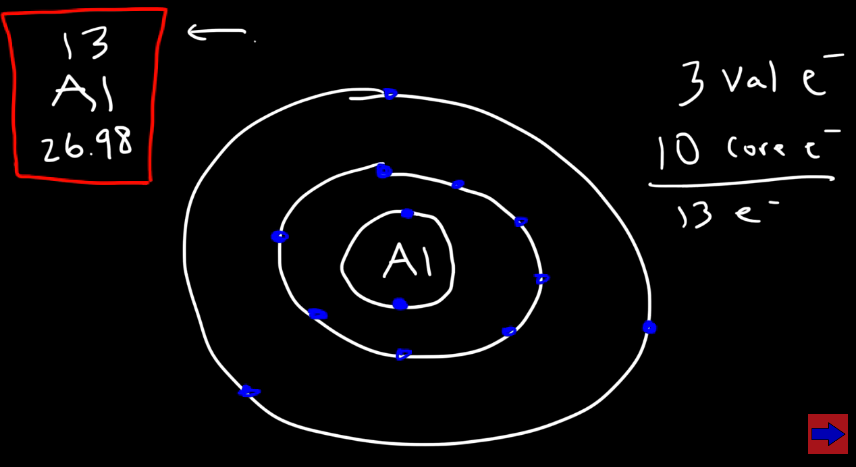
EX: Aluminum
Aluminum has 13 electrons. The first energy level can hold up to 2, the second 8, and the third 18, but we only need 3 electrons on the third shell to give us 13 electrons. So, Aluminum has 3 valence electrons (the electrons ONLY on the OUTERMOST shell of an atom) and 10 core electrons. (see image)
Figuring out Valence Electrons from Electron Configuration
(If you’re not sure how to do Electron Configuration, check flashcards in this file)
EX: Electron Configuration for Nitrogen and Aluminum:
Nitrogen: 1s2, 2s2, 2p3 (notice that the exponents add up to 7, because Nitrogen has 7 electrons)
In the highest energy level in this case, which is the second energy level, we have two sublevels 2s and 2p. We have a total of 5 valence electrons (In the outermost, or highest energy level) and 2 core electrons.
Aluminum: 1s2, 2s2, 2p6, 3s2, 3p1 . The highest energy level for aluminum is the third energy level (3). SO: Aluminum contains 3 valence electrons, and 10 core electrons (the rest of the electrons).
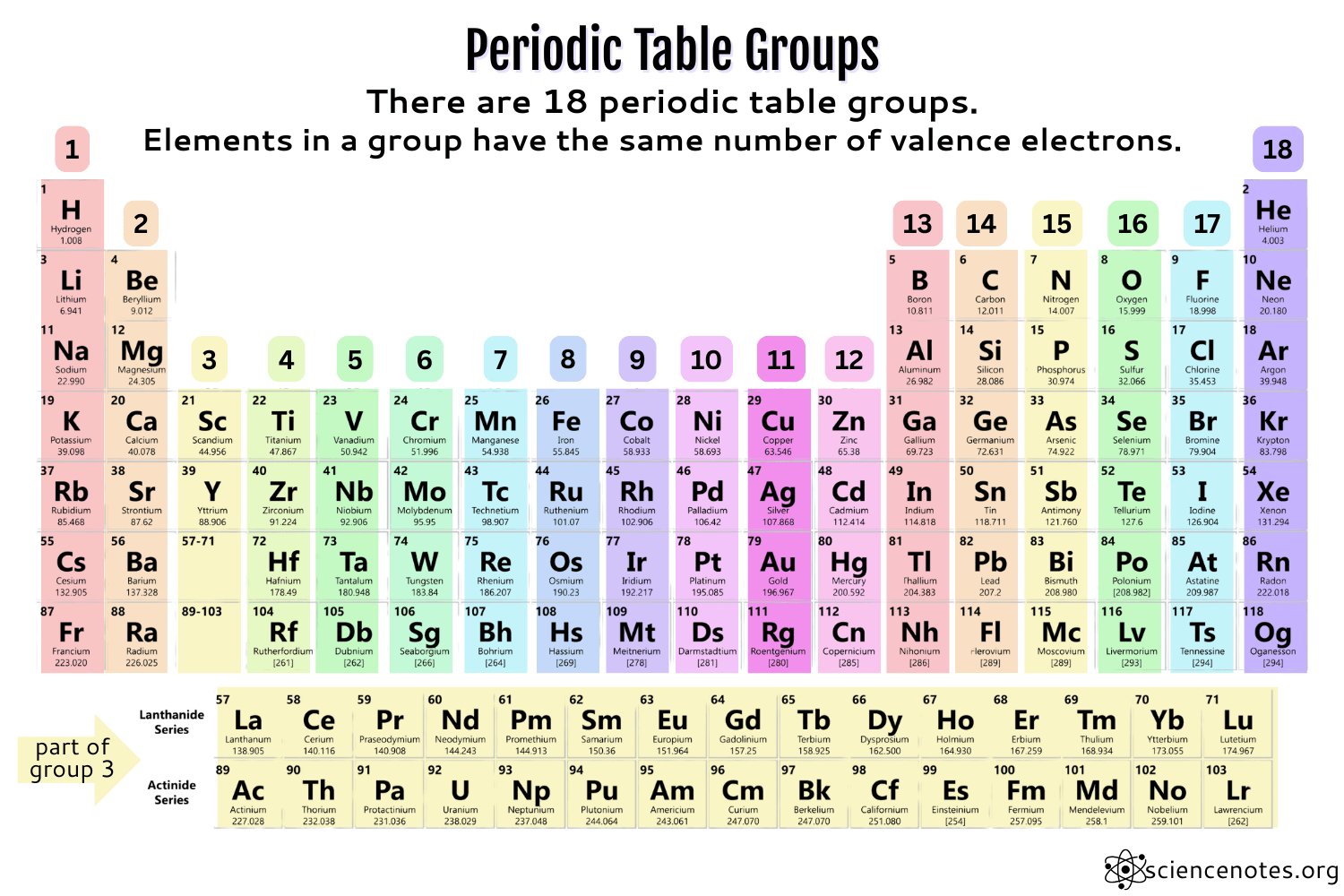
You can also find valence electrons from Groups on the Periodic Table
Group 1 have 1 valence electron. Group 2 have 2 valence electrons, (skip transition metals) Group 13 have 3 valence electrons. group 14 have 4 valence electrons, group 15 have 5 valence electrons, group 16 can hold up to 6 electrons, group 17 have 7 valence electrons and finally, group 18 have 8 electrons (except for Helium, which only has 2 electrons (because of its # of protons). (SEE IMAGE FOR GROUPS, look at number above the elements, which ever number is vertically above said element, is its group number).
To Find Core Electrons From Valence Electrons
Total Number of Electrons = Core Electrons + Valence Electrons
EX: Iodine has 53 electrons. Since it is in group 17, we know that it has 7 valence electrons. So, 53 = core electrons + 7. To solve, 53 - 7 = 46 core electrons.