Creep and Corrosion
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
What is creep?
Time-dependent permanent deformation under constant stress, usually at elevated temperature
When does creep typically occur in metals?
At temperatures > 0.3–0.4 Tm (melting temperature)
When does creep occur in ceramics?
At temperatures > 0.4–0.5 Tm (melting temperature)
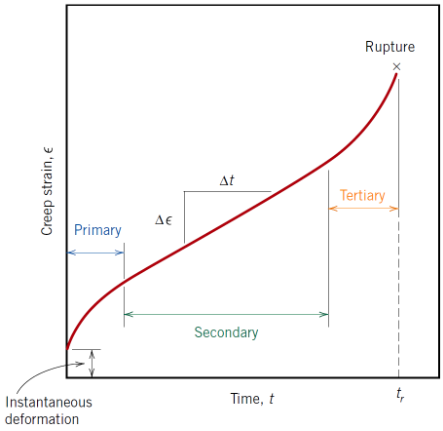
What are the 3 stages of creep?
• Primary
• Secondary
• Tertiary
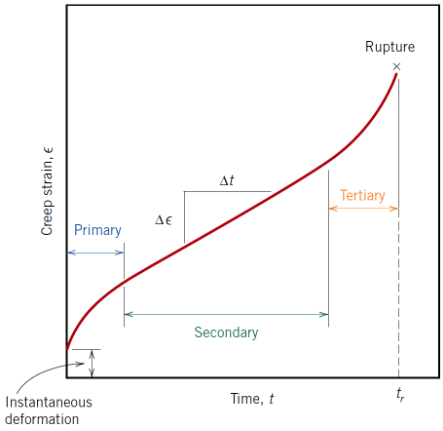
What characterises primary creep?
Continuously decreasing creep rate due to strain hardening
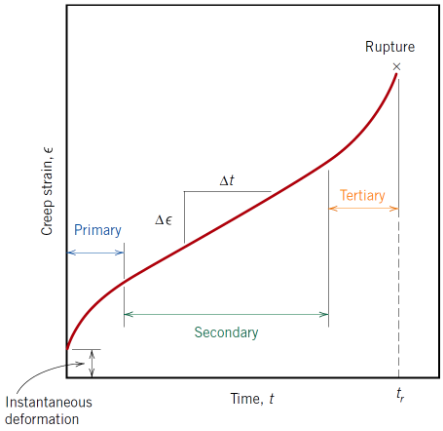
What characterises secondary creep?
Constant (steady-state) creep rate; most of a component’s service life is spent here
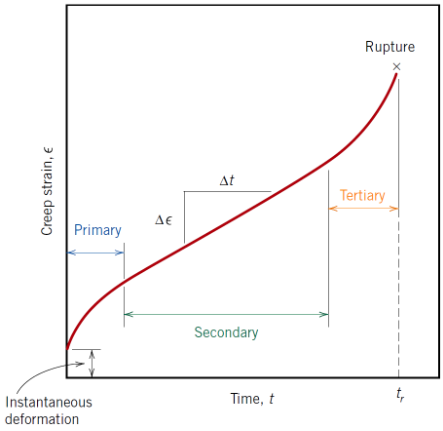
What characterises tertiary creep?
Accelerating creep rate leading to failure
Equation for Creep Rate
• 𝜀̇ = Creep rate
• K2 = Material constant
• QC = Activation energy for creep
• R = Gas constant
• T = Temperature
• n = Stress exponent (dependent on the mechanism)
(Creep is a diffusion controlled mechanism)
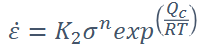
What is oxidation in corrosion?
Loss of valence electrons; occurs at the anode
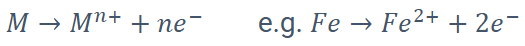
What is reduction in corrosion?
Gain of electrons; occurs at the cathode

What is standard electrode potential?
The voltage of a metal relative to the standard hydrogen electrode
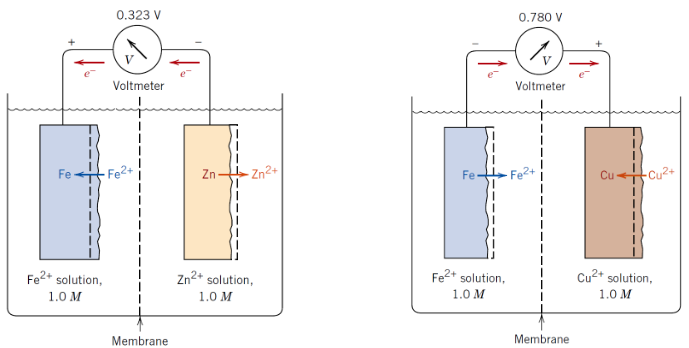
Why are electrode potentials important?
They predict which metal will corrode in a pair
What does the standard EMF series show?
Relative tendency of metals to oxidise (corrode)
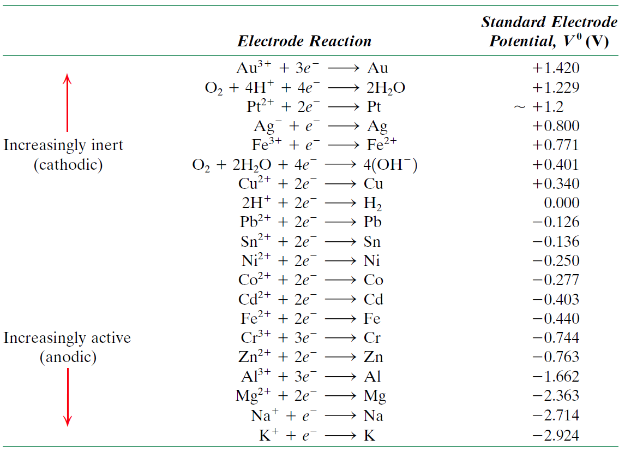
Which metals corrode more easily?
Those with more negative electrode potentials
Why does seawater affect corrosion behaviour?
Chloride ions change electrolyte conductivity and electrochemistry
What does the galvanic series predict?
Corrosion behaviour of metals in a specific environment
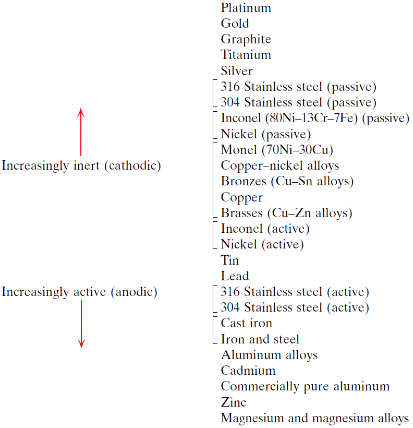
How can you tell which metal in a pairing is the anode?
The anode is the metal that is more reactive or has a lower (more negative) standard electrode potential (dictated by the standard emf series or the galvanic series)
How many main forms of corrosion are there?
8

What is uniform attack? (Corrosion)
Degree of corrosion is approximately uniform over the entire exposed surface
What is galvanic corrosion? (Corrosion)
Occurs when two different metals or alloys are electrically coupled while exposed to an electrolyte solution
What is crevice corrosion? (Corrosion)
The situation when corrosion occurs under crevices or other areas where there is localized depletion of oxygen
What is pitting? (Corrosion)
A type of localized corrosion in which pits or holes form from the top of horizontal surfaces
What is intergranular corrosion? (Corrosion)
Occurs preferentially along grain boundaries
What is selective leaching? (Corrosion)
The case wherein one element / constituent of an alloy is removed selectively by corrosive action
What is tribocorrosion? (Corrosion)
The combined action of chemical attack and mechanical wear
What is stress corrosion? (Corrosion)
The formation and propagation of cracks (and possible failure) resulting from a combination of corrosion and an applied tensile stress
Can corrosion ever be beneficial?
Yes, protective oxide layers can form (e.g. Stainless steel's "stainless" property comes from a thin, self-healing chromium oxide layer that forms and blocks further corrosion)
What limits oxidation rate in protective oxides?
Diffusion of metal and oxygen ions through the oxide
What is passivation?
Formation of a thin, protective, adherent oxide layer
Which metals passivate well?
Aluminium and Chromium
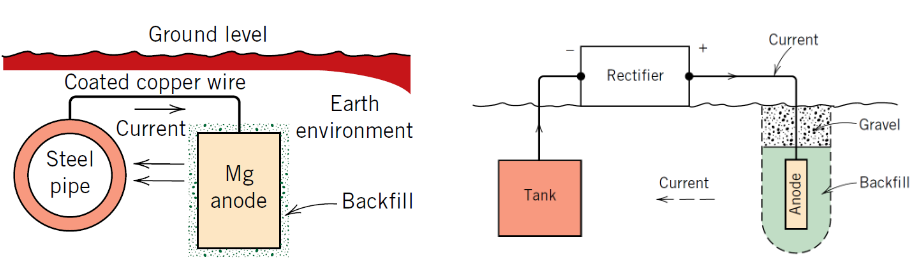
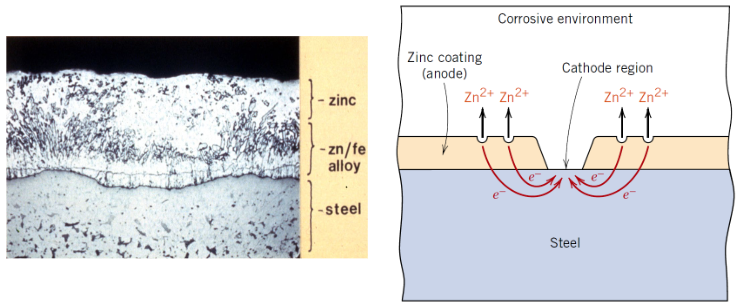
What is a sacrificial anode?
A more reactive metal that corrodes instead of the protected metal
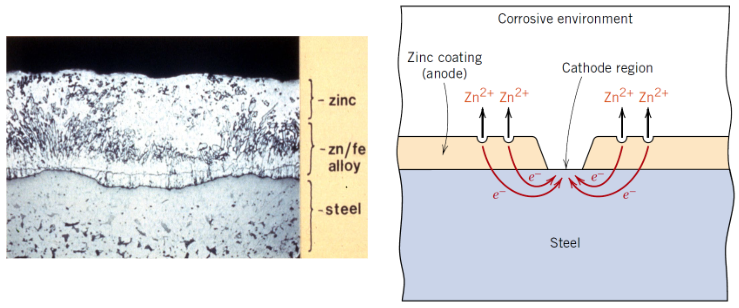
Which metals are commonly used as sacrificial anodes?
• Zinc
• Magnesium
• Aluminium
Why does zinc protect steel?
Zinc has a more negative electrode potential than iron
What is Impressed Current Cathodic Protection (ICCP) ?
External current forces the structure to act as a cathode