17 Spectroscopy
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
what happens when an organic compound is placed in a mass spectrometer
it loses an electron and forms a positive ion - the molecular ion
what does the mass spectrometer detect
the mass-to-charge (m/z) of the molecular ion
this gives the molecular mass of the compound
formula for formation of the molecular ion of propan-1-ol
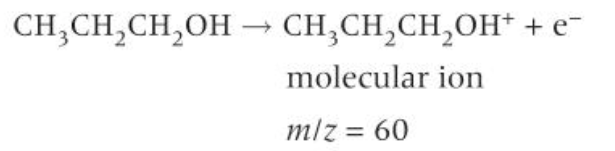
where is the M+ peak on a mass spectrum
the clear peak at the highest m/z value on the RHS of the mass spectrum
what is the small peak one unit after the M+ peak
the M+1 peak
this exists because 1.1% of carbon is present as the carbon-13 isotope
a compound might contain an atom of 13C so its molecular mass will be m+1
fragmentation def
when some molecular ions break down into smaller pieces known as fragments in the mass spectrometer
what are the other peaks on a mass spectrometer caused by
fragment ions formed from the breakdown of the molecular ion
simplest fragmentation equation example of propan-1-ol
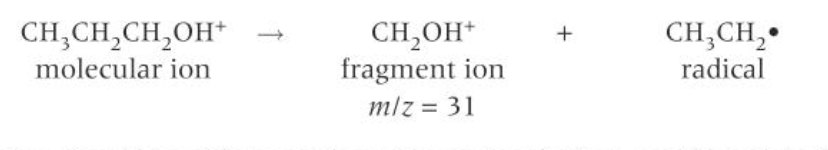
what is formed in fragmentation
a positive ion and a radical
any pos ions will be detected by the mass spectrometer but the uncharged radical are undetected
how can mass spectra help to identify which isomer/fragment ions are present
the mass spectrum of each compound is unique as molecules all fragment in slightly different ways depending on structure
even if two molecules have the same molecular mass and the same molecular ion peak, the fragment ions found in the spectrum may be different
what are the two types of vibration atoms in molecules can do
bending or stretching
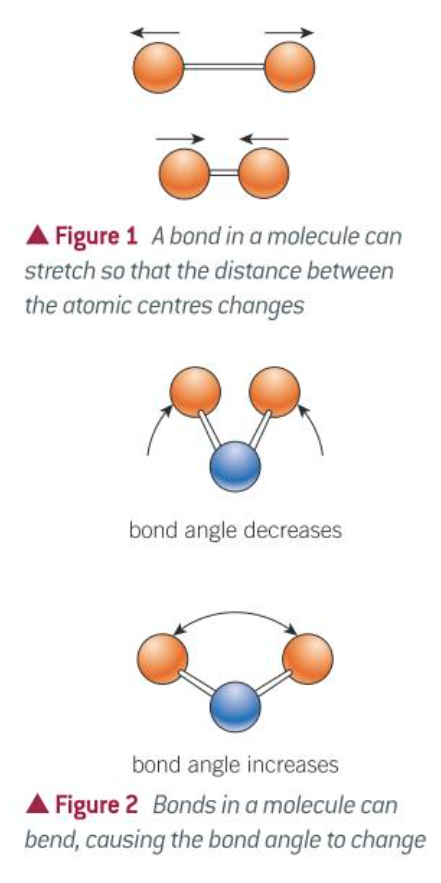
what does the amount a bond stetches or bends depend on
the mass of the atoms in the bond: heavier atoms vibrate more slowly than lighter atoms
the strength of the bond: stronger bonds vibrate faster than weaker bonds
what’s a feature of bonds to do w radiation
any particular bond can only absorb radiation that has the same frequency as the natural frequency of the bond
which gases absorb longer-wavelength IR (infrared) radiation and why
water vapour, carbon dioxide, methane (greenhouse gases)
because they have the same frequency as the natural frequency of the bonds
eventually the vibrating bonds re-emit this energy as radiation that increases the temperature of the atmosphere close to the Earth’s surface
what is infrared spectroscopy used for
to identify the functional groups present in organic molecules
what is the fingerprint region of a infrared spectrum
the many peaks below 1500 cm-1
contains unique peaks that can be used to identify particular molecule under investigation (using computer software)
IF spectrum of an alcohol group
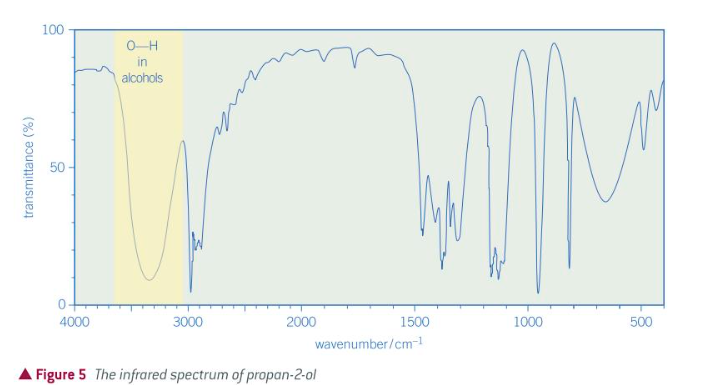
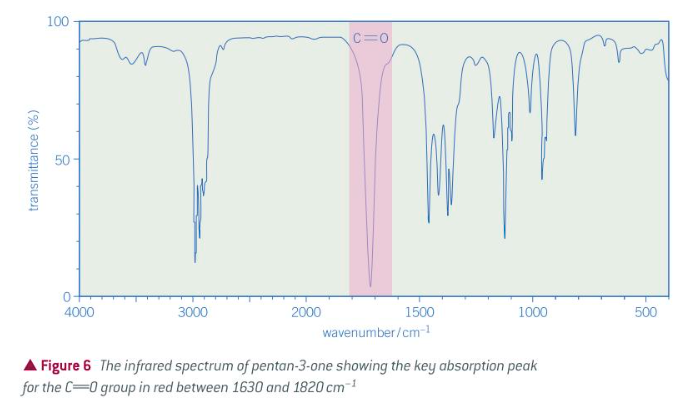
IF spectrum of an aldehyde/ketone group
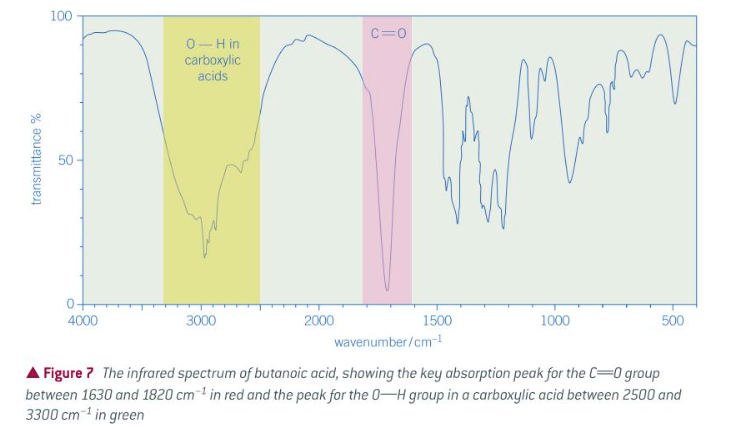
IF spectrum of a carboxylic acid
applications of infrared spectroscopy
identifying pollutants (remote sensors analyse IR spectra of vehicle emissions to detect and measure CO, CO2 and hydrocarbons in busy town centres/by motorways to monitor localised pollution)
breathalysers (pass a beam of IR radiation through captured breath to detect IR absorbance of compounds in breath, characteristic bonds present in ethanol detected. the more IR radiation absorbed, higher reading, more ethanol in breath)
typical sequence for identification of organic compounds
elemental analysis (use of percentage composition data to determine empirical formula)
mass spectrometry (use of molecular ion peak from a mass spectrum to determine molecular mass, use of fragment ions to identify sections of a molecule)
infrared spectrometry (use of absorption peaks from an infrared spectrum to identify bonds and functional groups present)