Structure and Bonding
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
47 Terms
The way that atoms are bound to each other to make molecules, has great effect on the WHAT and WHAT properties of matter
The way that atoms are bound to each other to make molecules has great effect on the PHYSICAL and CHEMICAL properties of matter
Graphite and diamond are both composed of WHAT atom, with a different way of WHAT
Graphite and diamond are both composed of CARBON atom, with a different way of BONDING

Different elements have different desires for WHAT an electron or WHAT an electron from/to their valence shell
Different elements have different desires for LOSING an electron or GAINING an electron from/to their valence shell
When two atoms are brought together, their WHAT are affected
When two atoms are brought together, their VALENCE ELECTRONS are affected
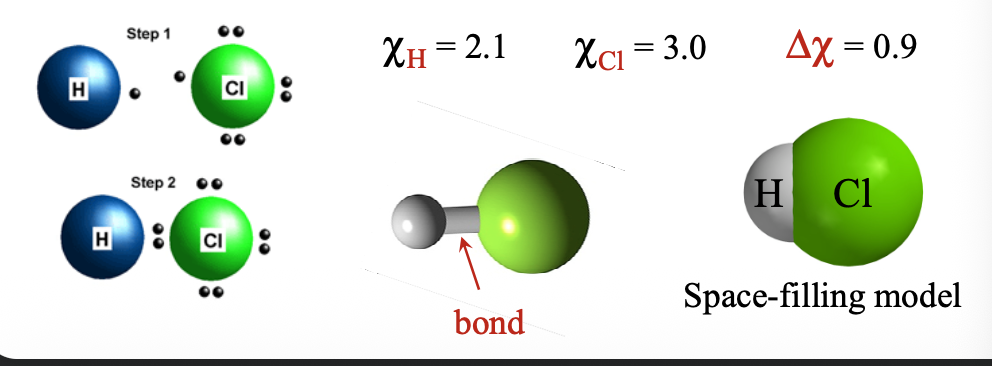
Electronegativity (x or EN) is the desire of one atom for the WHAT of the neighbouring WHAT
Electronegativity (x or EN) is the desire of one atom for the VALENCE ELECTRONS of the neighbouring ATOMS
Electronegativity cannot be measured WHAT
Electronegativity cannot be measured DIRECTLY
The difference in electronegativity (Δχ) of atoms determines how the WHAT are distributed when two atoms WHAT
The difference in electronegativity (Δχ) of atoms determines how the VALENCE ELECTRONS are distributed when two atoms COME TOGETHER
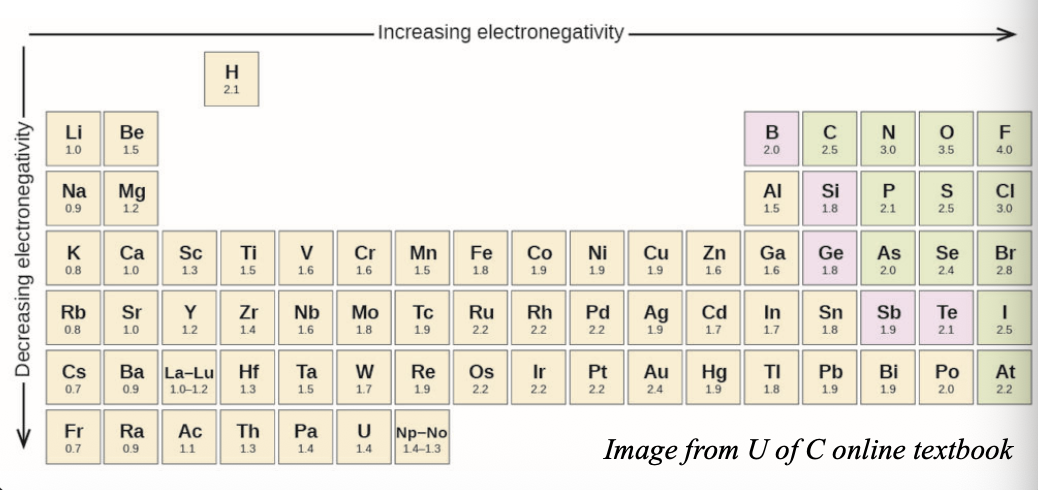
Δχ can be used to predict the WHAT
Δχ can be used to predict the NATURE OF BONDING
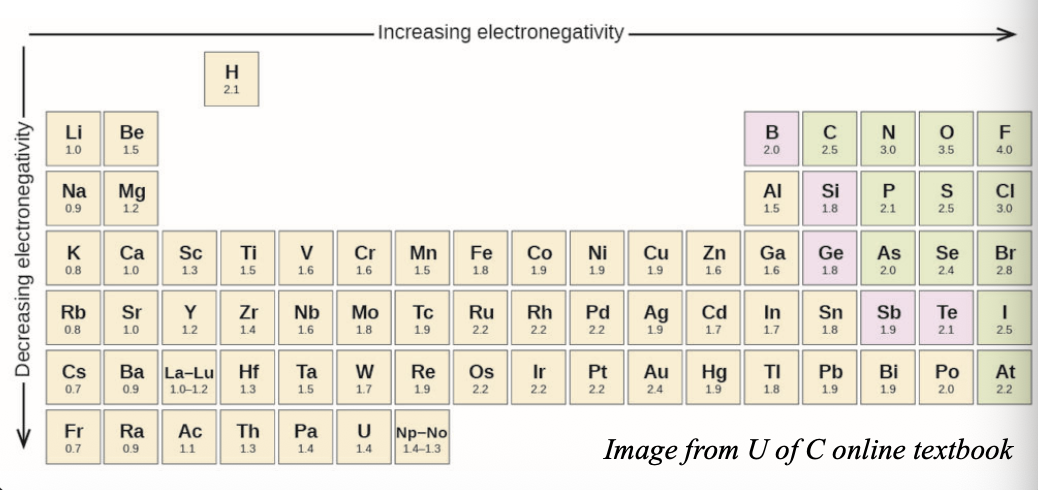
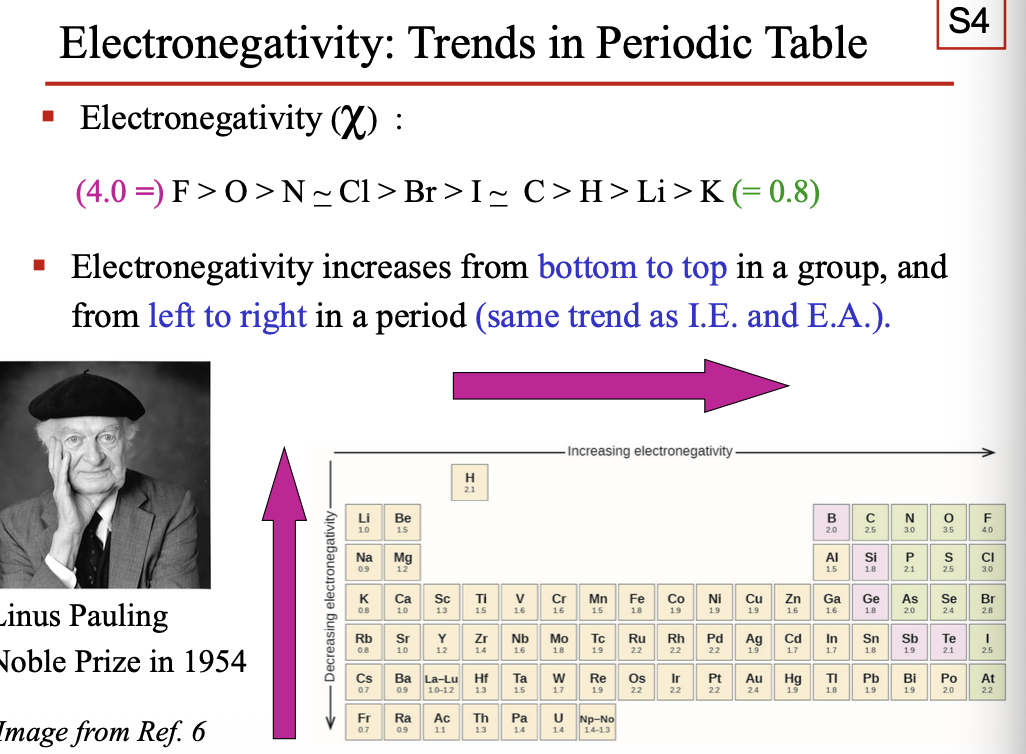
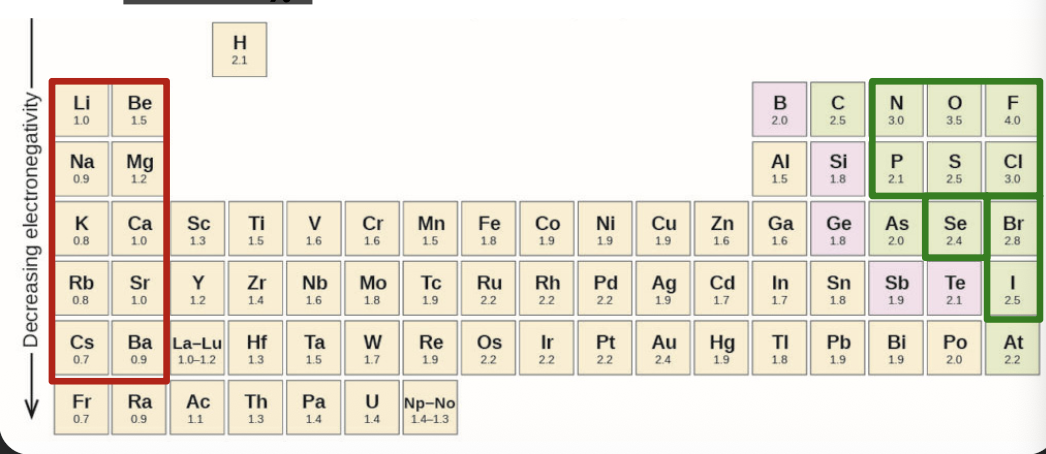
Electronegativity increases from WHAT to WHAT in a group, and from WHAT to WHAT in a period (same as I.E and E.A)
Electronegativity increases from BOTTOM to TOP in a group, and from LEFT to RIGHT in a period (same as I.E and E.A)
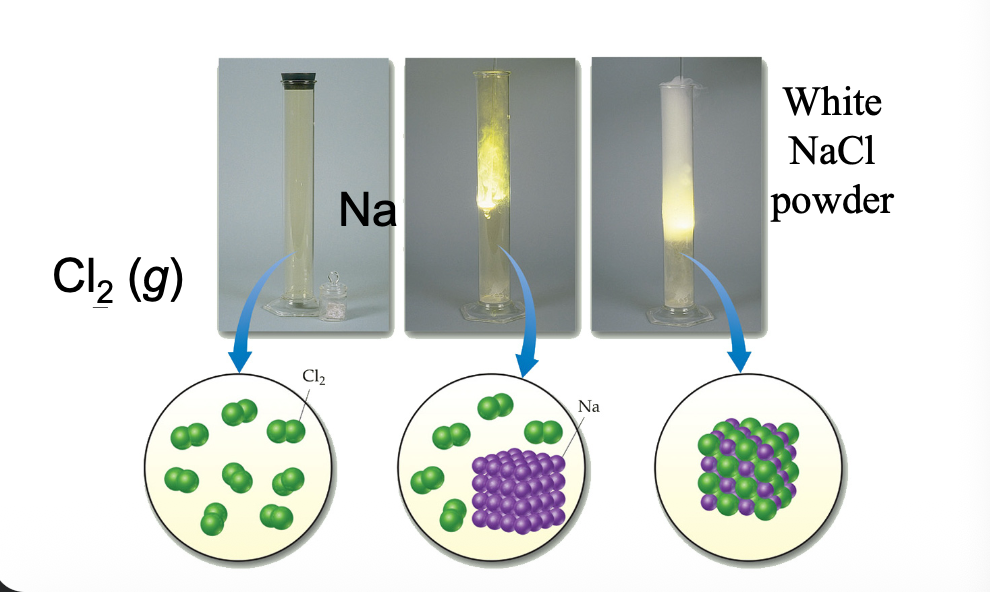
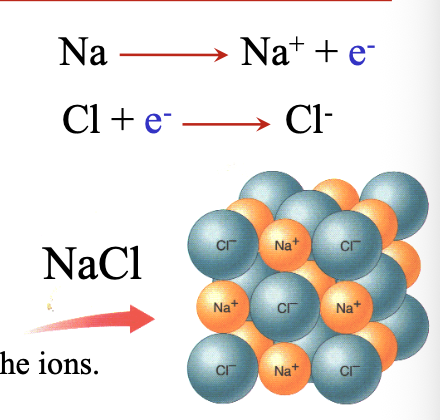
Reaction of Cl2 gas with Na metal is WHAT, forming NaCl
Reaction of Cl2 gas with Na metal is EXOTHERMIC, forming NaCl
Metals easily WHAT electrons (WHAT I.E) while, non-metals have high WHAT for electrons (WHAT E.A)
Metals easily LOSE electrons (LOW I.E), while non-metals have high AFFINITY for electrons (HIGH E.A)

When atoms with large difference in electronegativity Δχ > WHAT come together, WHAT are transferred and WHAT are formed
When atoms with large difference in electronegativity Δχ > 1.8 come together, VALENCE ELECTRONS are transferred and IONS are formed

Curved arrows can be used as a tool to show the WHAT
Curved arrows can be used as a tool to show the ELECTRON TRANSFER
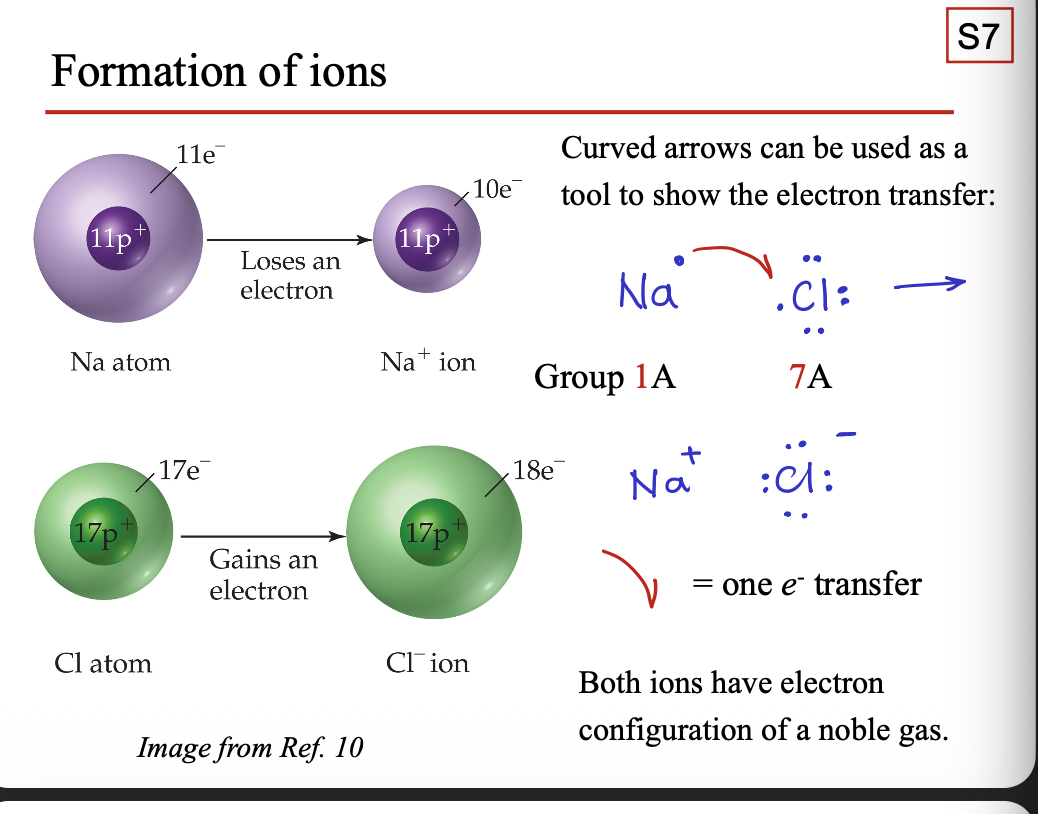
Ionic bonding
ELECTROSTATIC FORCES (attractions) between positive and negative ions that are closely packed together in an IONIC SOLID COMPOUND
Ionic bonding occurs in HOW MANY dimensions and is WHAT (not just between 2 atoms; not in one direction in space)
Ionic bonding occurs in THREE dimensions and is DELOCALIZED (not just between 2 atoms; not in one direction in space)
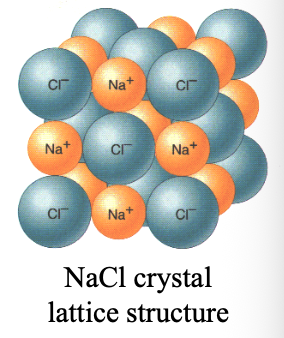
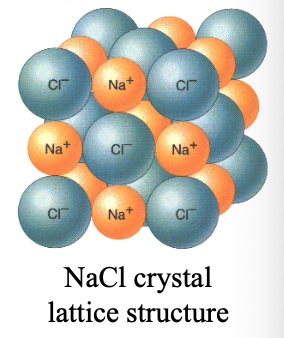
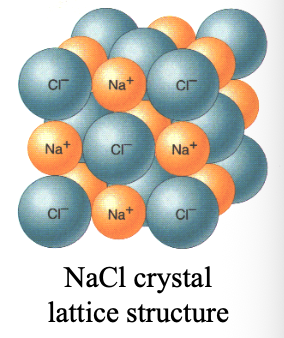
WHAT of ionic compounds (ionic WHAT) are repeated arrangements of cations and anions in 3D. forming a WHAT structure
CRYSTALS of ionic compounds (ionic CRYSTALS) are repeated arrangements of cations and anions in 3D. forming a LATTICE structure
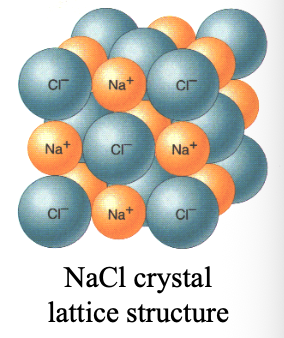
In ionic crystals, attractive forces (+, -) are WHAT and repulsive forces (+,+)/(-,-) are WHAT, holding ions together
In ionic crystals, attractive forces (+, -) are MAXIMIZED and repulsive forces (+,+)/(-,-) are MINIMIZED, holding ions together
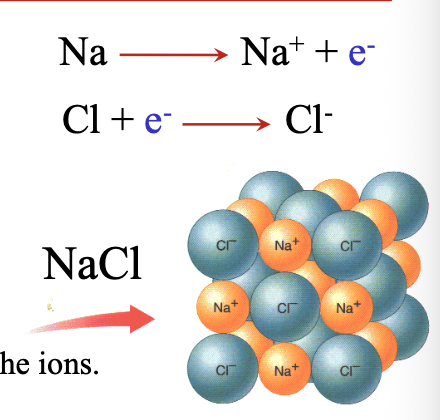
Steps for transferring electrons:
Step 1: Transfer of WHAT electrons from sodium atom to chlorine atom, forming WHAT and WHAT ions
Step 2: Ionic bonding between WHAT and WHAT ions that are closely packed together
Steps for transferring electrons:
Step 1: Transfer of VALENCE electrons from sodium atom to chlorine atom, forming Na+ and Cl- ions
Step 2: Ionic bonding between Na+ and Cl- ions that are closely packed together
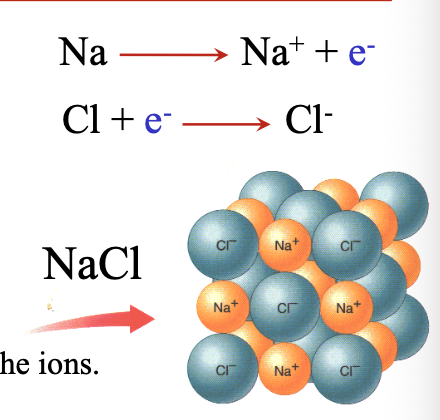
The formication of the WHAT stabilizes the ions
The formication of the LATTICE stabilizes the ions
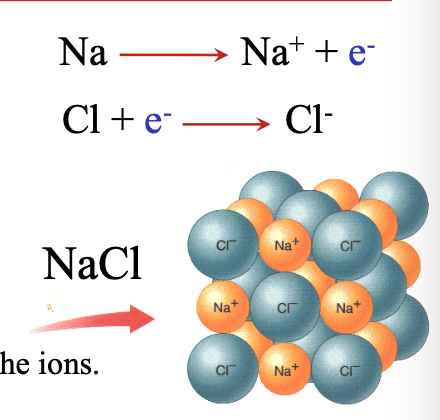
Ionic chemical formulas are written as WHAT formula (smallest whole ratio of WHAT to WHAT)
Ionic chemical formulas are written as EMPIRICAL formula (smallest whole ratio of CATION to ANION)
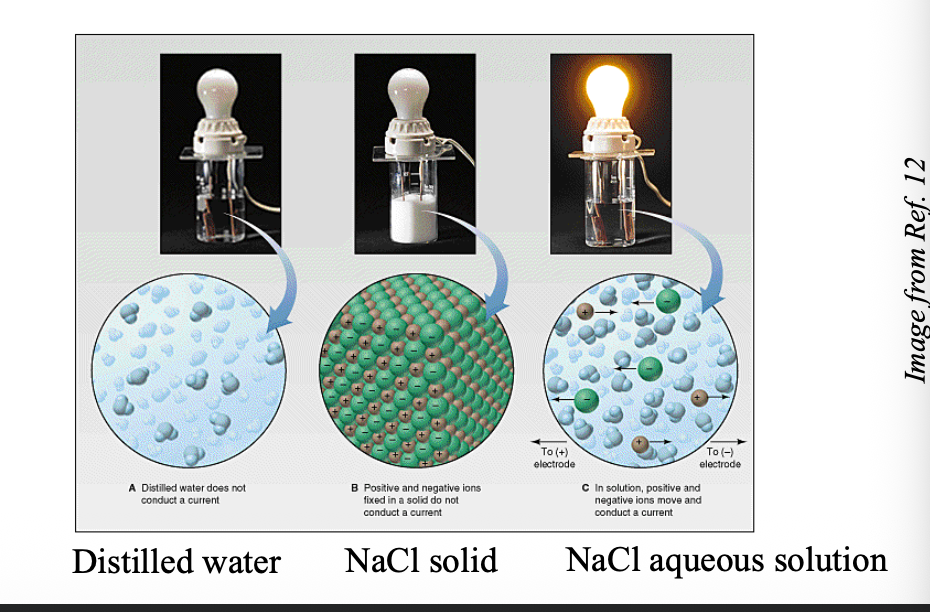
There is no WHAT molecule in solid state or aqueous solution
There is no NaCl molecule in solid state or aqueous solution
When dissolved in water, hydrated ions can conduct WHAT
When dissolved in water, hydrated ions can conduct ELECTRICITY
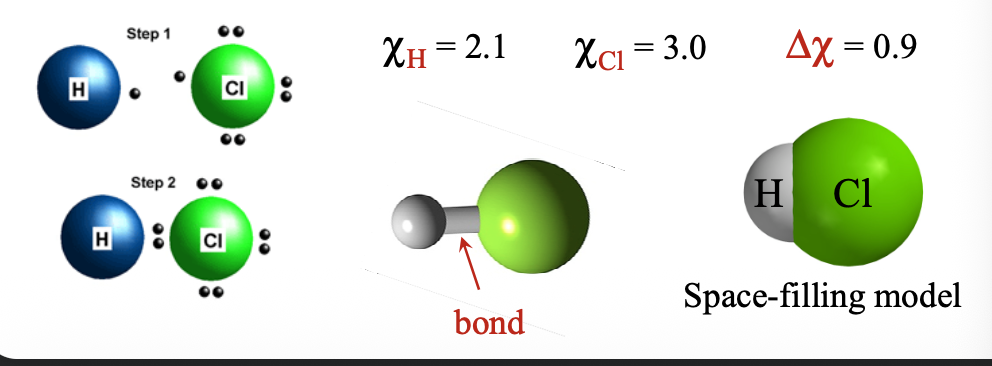
When two atoms both with fairly similar WHAT and WHAT and small WHAT difference (Δχ - WHAT-WHAT) come together, no valence electron is transferred between the atoms; ions not WHAT
When two atoms both with fairly similar E.A. and I.E. and small ELECTRONEGATIVITY difference (Δχ - 0-1.8) come together, no valence electron is transferred between the atoms; ions not FORMED
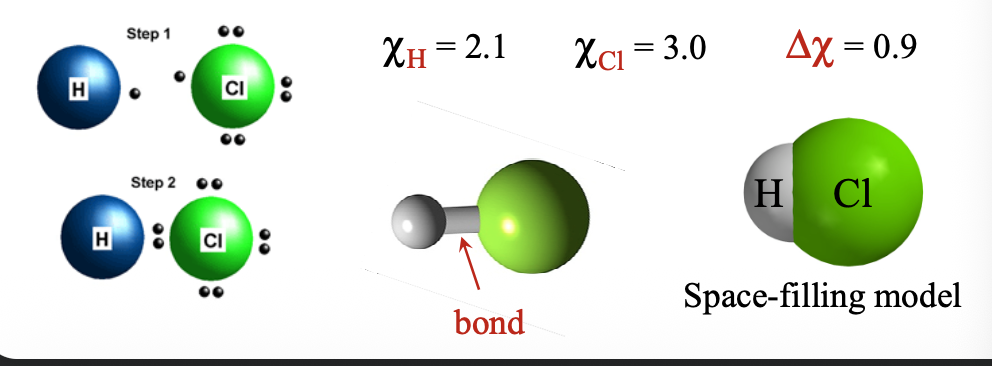
These atoms can WHAT their valence electrons and form WHAT
These atoms can SHARE their valence electrons and form BONDS
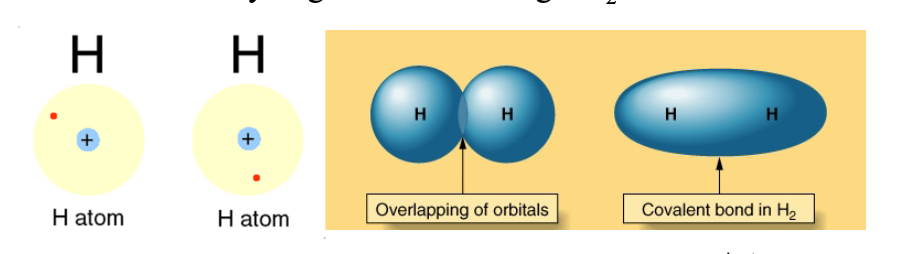
Covalent bonds are WHTA forced creating WHAT molecules
Covalent bonds are LOCALIZED forced creating INDIVIDUAL molecules
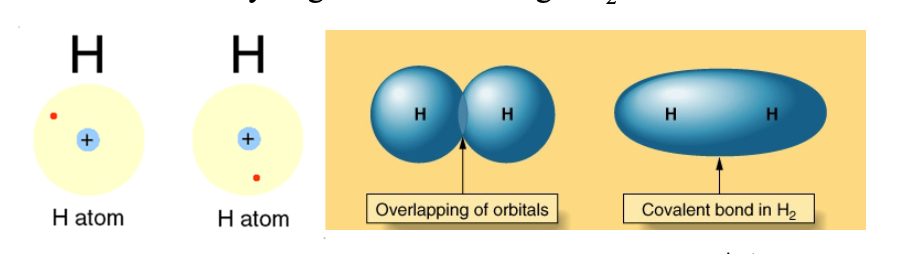
As the two H atoms get closer, the electron density on each atom is attracted by both WHAT, so the probability increases to find the electrons between the WHAT
As the two H atoms get closer, the electron density on each atom is attracted by both NUCLEI, so the probability increases to find the electrons between the TWO ATOMS
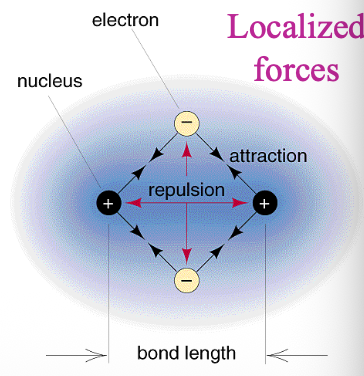
The atoms cannot get very close to each other, due to the WHAt forces between two positively charged nuclei
The atoms cannot get very close to each other, due to the REPULSION ELECTROSTATIC forces between two positively charged nuclei
Determining the type of bonding between two atoms:
Physical properties provide crucial evidence for the type of bonding
Ionic compounds:
Form WHAT salt with very high WHAT, due to WHAT between ions
Conduct WHAT when dissolved in water, forming WHAT ions
Determining the type of bonding between two atoms:
Physical properties provide crucial evidence for the type of bonding
Ionic compounds:
Form CRYSTALLINE salt with very high MELTING POINTS, due to ELECTROSTATIC ATTRACTIONS between ions
Conduct ELECTRICITY when dissolved in water, forming SOLVATED ions
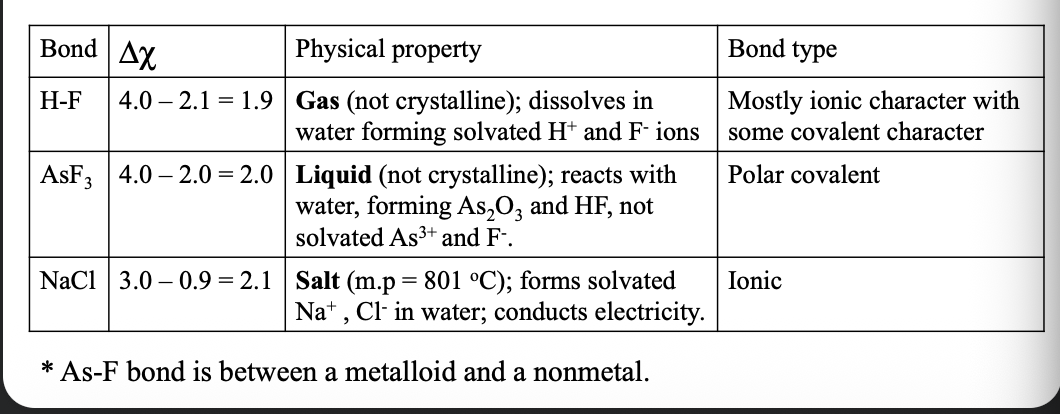
Determining the type of bonding between two atoms:
Bonds between one metal and one non-metal is often WHAT, while bonds between two non-metals are generally WHAT
The position of the elements involved in the periodic table overrides the WHAT, when deciding about the type of bonding
Determining the type of bonding between two atoms:
Bonds between one metal and one non-metal is often IONIC, while bonds between two non-metals are generally COVALENT
The position of the elements involved in the periodic table overrides the ΔEN (or Δχ), when deciding about the type of bonding
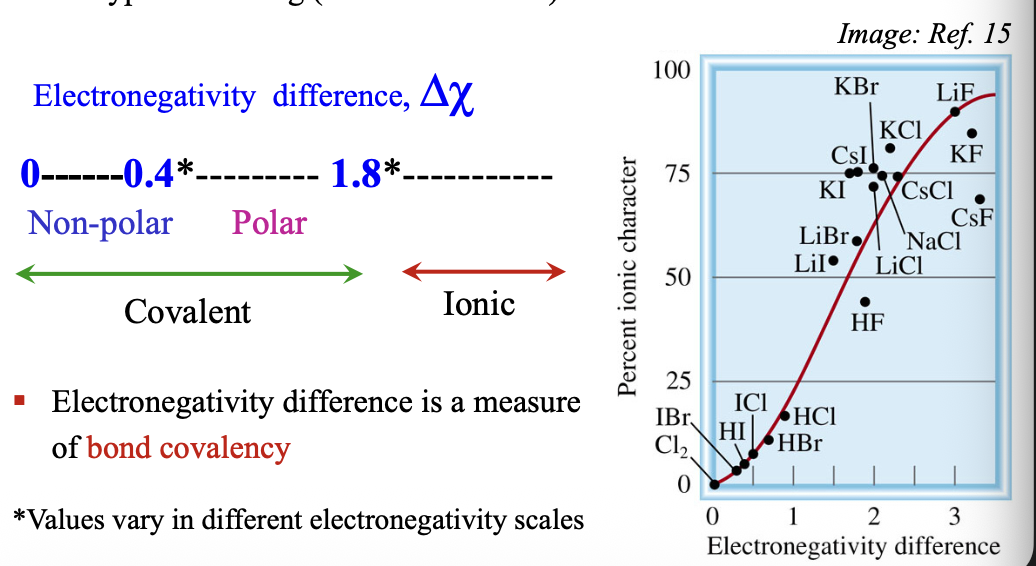
Determining the type of bonding between two atoms:
Electronegativity values can be used to predict (WHAT-WHAT% of the time) the type of WHAT (covalent vs ionic) that occurs between atoms
Electronegativity difference is a measure of WHAT
Determining the type of bonding between two atoms:
Electronegativity values can be used to predict (70-80% of the time) the type of BONDING (covalent vs ionic) that occurs between atoms
Electronegativity difference is a measure of BOND COVALENCY
Atoms with different electronegativity values will share bonding electrons WHAT
Atoms with different electronegativity values will share bonding electrons UNEQUALLY
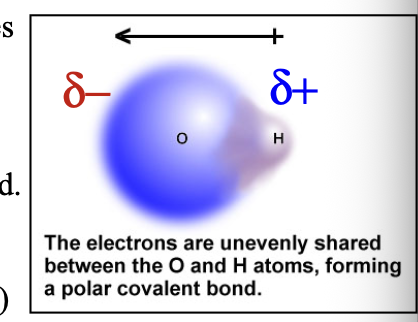
Bond dipole shows WHAT distribution (sharing) of electron density within a WHAT bond
Bond dipole shows UNBALANCED distribution (sharing) of electron density within a COVALENT bond
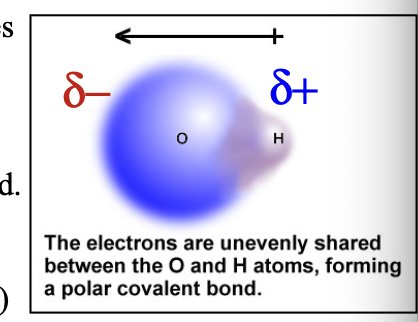
Bond dipole shown with delta (δ) indicated that a WHAT charge (less than full +1 and -1) has arisen: δ(+) and δ(-) are the two WHAT
Bond dipole shown with delta (δ) indicated that a PARTIAL charge (less than full +1 and -1) has arisen: δ(+) and δ(-) are the two POLES
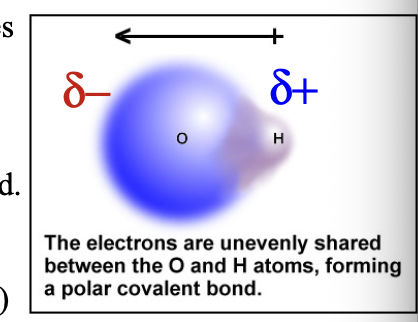
Partial negative δ(-) charge is assigned to the more WHAT atom
Partial negative δ(-) charge is assigned to the more ELECTRONEGATIVE atom
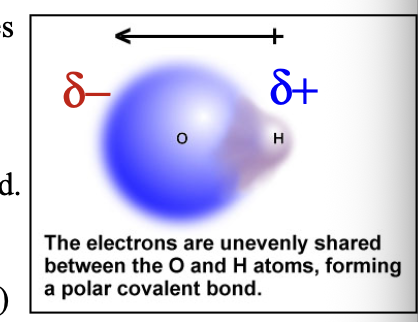
Such a bond is called a WHAT bond
Such a bond is called a POLAR COVALENT bond
For polar covalent bonds WHAT < Δχ < or = WHAT
For polar covalent bonds 0.4 < Δχ < or = 1.8
The greater the difference in WHAT, the more WHAT is the bond
The greater the difference in ELECTRONEGATIVITY, the more POLAR is the bond
Larger arrow = larger WHAT (Δχ) of the two atoms sharing electrons in a WHAT bond
Larger arrow = larger DIFFERENCE in ELECTRONEGATIVITY (Δχ) of the two atoms sharing electrons in a POLAR COVALENT bond
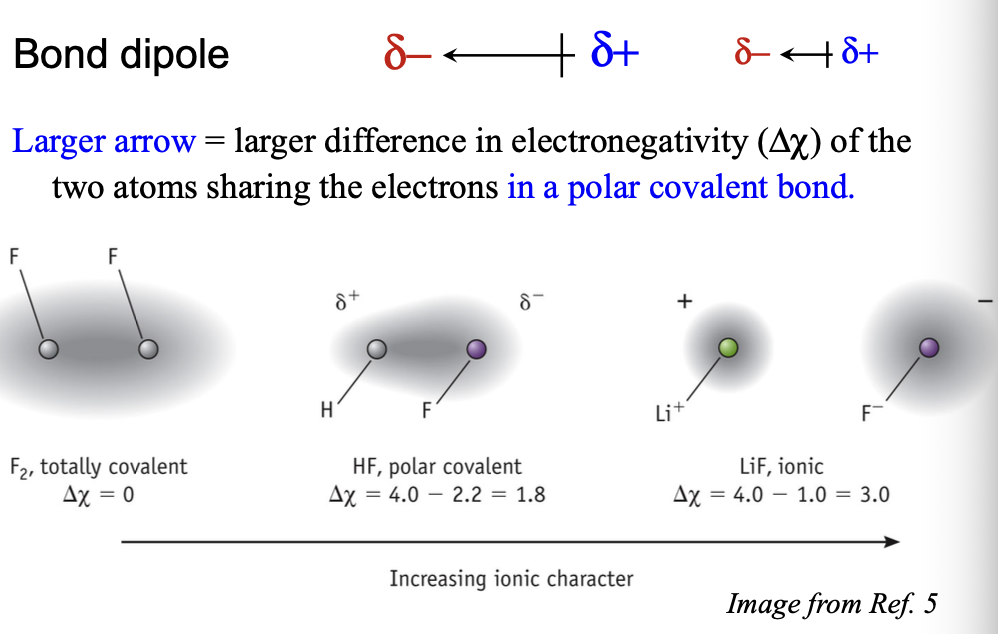
The difference in electronegativity of C and H in C-H bond in Δχ = WHAT
The difference in electronegativity of C and H in C-H bond in Δχ = 0.4
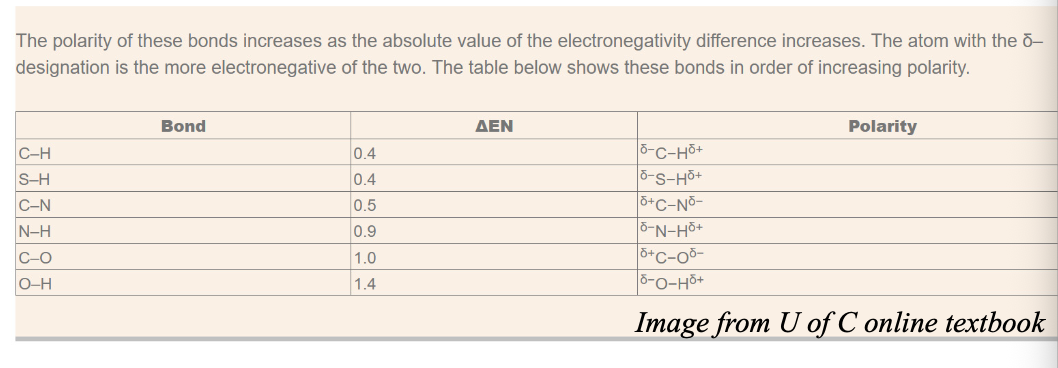
When examining physical or chemical changes, this polarity difference is NOT WHAT and the behaviour of chemical species indicates that
When examining physical or chemical changes, this polarity difference (for C-H) is NOT SIGNIFICANT and the behaviour of chemical species indicates that
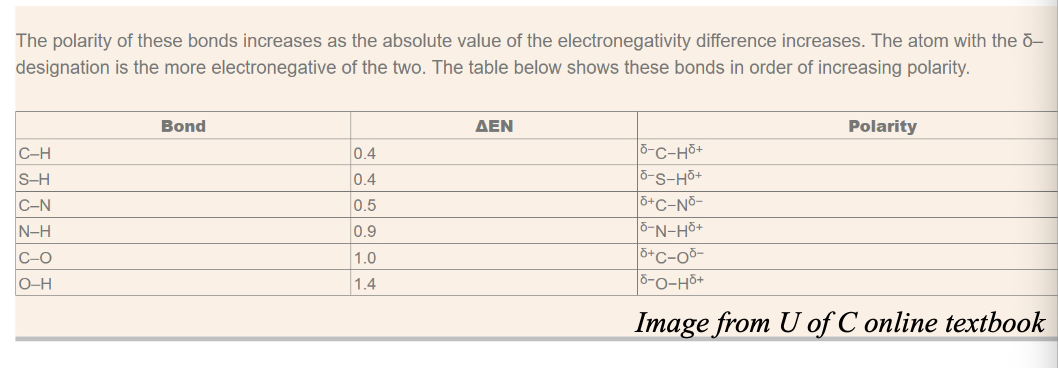
When Δχ = WHAT or greater, the bond polarity becomes “significant” and we assign WHAT on the atoms with WHAT bonds
When Δχ = 0.5 or greater, the bond polarity becomes “significant,” and we assign PARTIAL CHARGES (δ+ and δ-) on the atoms with COVALENT bonds
Compounds like sodium nitrite, Na(NO2) are both WHAT and HWAT
Compounds like sodium nitrite, Na(NO2) are both IONIC and COVALENT
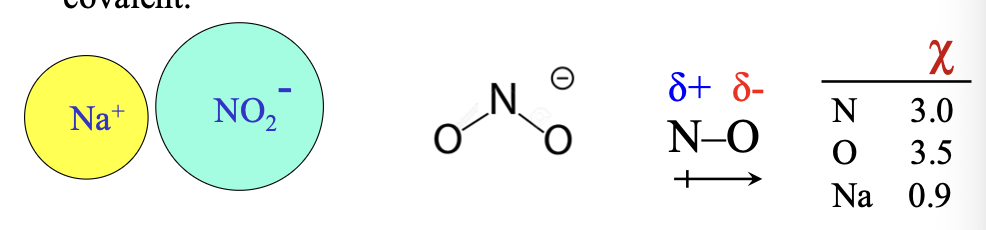
Electronegativity (χ): the desire of one atom for the WHAT of the neighbouring atoms
Electronegativity (χ): the desire of one atom for the VALENCE ELECTRONS of the neighbouring atoms
Δχ can be used as a tool to predict the nature of WHAT
Δχ can be used as a tool to predict the nature of BONDING
When Δχ > WHAT between two atoms, valence electrons are WHAT and WHAT are formed
When Δχ > 1.8 between two atoms, valence electrons are TRANSFERRED and IONS are formed
When WHAT < Δχ < or = WHAT between two atoms, valence electrons are WHAT = WHAT bonds (WHAT between two atoms)
When 0 < Δχ < or = 1.8 between two atoms, valence electrons are SHARED = COVALENT bonds (LOCALIZED between two atoms)
When 0 < or = Δχ < 0.4; WHAT covalent bonds
When 0 < or = Δχ < 0.4; NON-POLAR covalent bonds