Functional Polymers in Flexible Electronics
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
What is a Polymer?
A polymer is defined as a macromolecule of high molecular weight formed by the repeated combination of several simple molecules called monomers of one or more type through covalent bonds.
Eg. Polyethylene, nylon, PVC (polyvinyl chloride), Teflon, polyester, bakelite, etc.
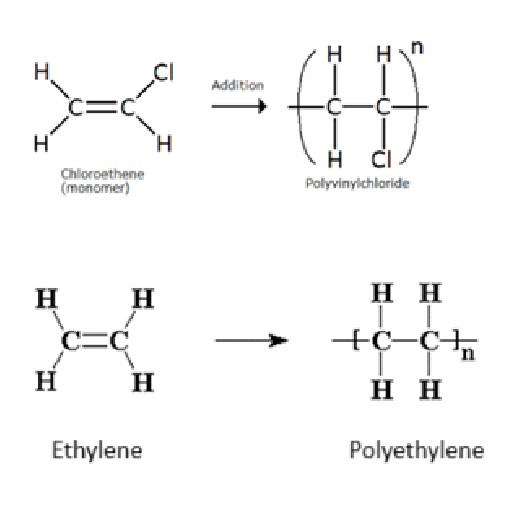
What is a Monomer?
Simple/small molecules which combine with each other to form polymer. Eg.Ethylene, vinyl chloride, butylene, chloroprene etc.
What is Polymerization?
The chemical reaction in which monomer is converted into polymer.
What is Degree of Polymerization?
The number of repeating units in a polymer
What is Functionality?
The number of bonding sites in a monomer molecule.
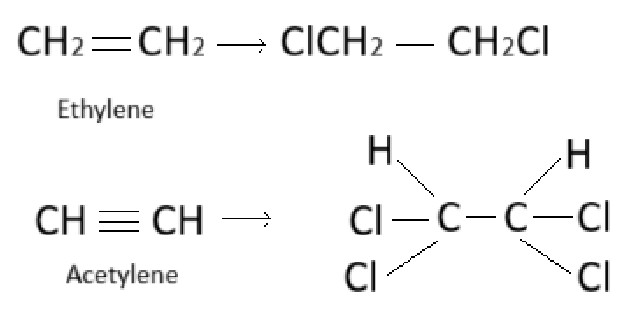
For example, ethylene can add two molecules of hydrogen or halogen. Hence, it is bifunctional (functionality two).
Similarly, acetylene has a functionality of four (tetrafunctional), as it can react with four atoms of hydrogen or halogen
Why is Average Molecular Mass of polymer taken?
During the formation of polymers, different polymers have different degrees of polymerization.
Thus, molecular masses of individual macromolecules in a particular sample of polymer are different. Hence, an average value of the molecular mass is taken.
What are the ways in which Average Molecular Mass can be calculated?
Number – average molecular mass (Mn)
Mass – average molecular mass (Mw)
What is Number Average Molecular weight (Mn)?
It is determined by measurement of colligative properties such as depression in freezing point, elevation of boiling point, osmotic pressure and lowering of vapour pressure.
It is defined as the total mass of all the molecules in a polymer sample divided by total number of molecules present.
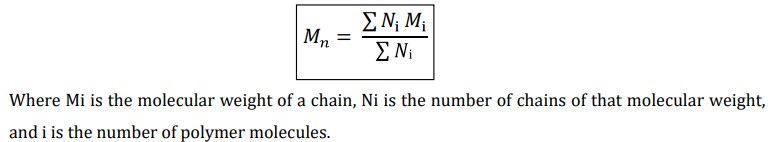
What is Weight average molecular mass (Mw)?
It is obtained by light scattering and ultra centrifugation techniques, which measures properties that depends on the molecular size.
The weight average molecular mass is always greater than number average molecular mass
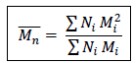
What is Poly Dispersity Index (PDI)?
In order to obtain the idea of homogeneity of a polymer, the term poly dispersity index is used.
It is the ratio of mass average molecular mass to the number average molecular mass.
PDI= Mw/Mn
What is the significance of PDI?
Monodisperse or Natural Polymers whose molecules have same or narrow range of molecular masses. PDI is usually = 1
Polydisperse or Synthetic Polymers which have wide range of molecular masses. PDI > 1
What are Conducting Polymers?
An organic polymer with highly delocalized pi-electron system, having electrical conductance of the order of a conductor is called a conducting polymer.
What is the Key structural feature of conducting polymer?
Linear structure with alternate single and double bonds, i.e., extensive pi conjugation in the backbone.
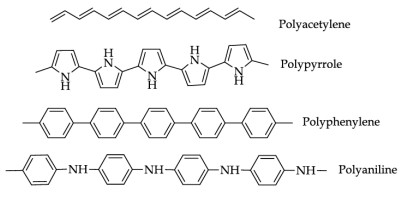
What are some examples of Conducting Polymers?
The electrical conductivity of polyacetylene can be increased to 13 folds by doping with electron acceptors and donors.
The electrical conductivity of doped polyacetylene (105 sm-1) is remarkable to Teflon (10-8 sm-1) but is marginally lower than copper (108 sm-1).
How are conducting polymers synthesized?
The conducting polymers are synthesized by doping, in which charged species are introduced in organic polymers having pi-backbone.
The important doping reactions are
Oxidative doping (p-doping)
Reductive doping (n-doping)
Protonic acid doping.
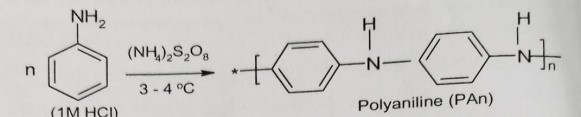
How is Polyaniline synthesized?
It is obtained by polymerization of aniline dissolved in 1M HCl at 3-4°C in presence of ammonium persulphate as an initiator.
What are some Properties and Applications of conducting polyaniline?
Tremendous applications would exist as they are flexible, ease of fabrication, stability, ease of processability
It is used as an electrode in rechargeable solid state batteries (Li-PAn batteries). (This is due to its reversible electrochemical response during anodic oxidation and cathodic reduction).
It is used in anodic passivation of metals as a means of corrosion control.
Polyaniline exhibits different colour in oxidized and reduced forms and hence it is used in humidity sensors, gas sensors, radiation sensors.
Due to same property it is also used in electrochromic display windows.
It is used as conductive track on PCBs.
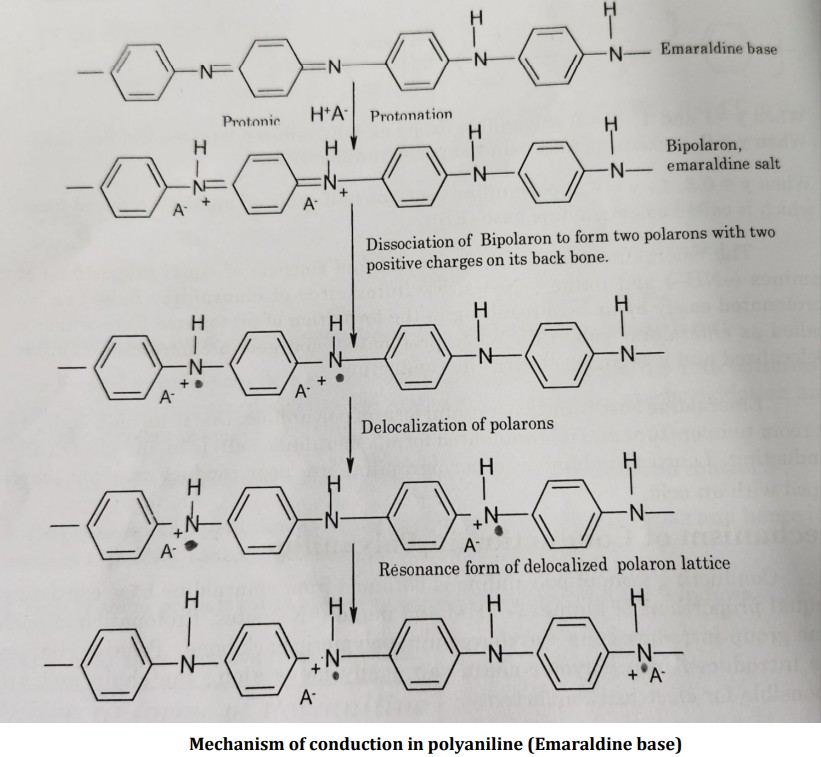
Explain Mechanism of Conduction in Polyaniline
Conducting form of polyaniline is obtained from emaraldine base consisting of equal proportions of amines (-NH-) and imine (=N-) sites.
Protonation of each imine group introduces one +ve charge into polymeric back bone.
Positive charges once introduced into polymer chain can easily move along the chain and are responsible for electrical conductivity.
Emaraldine base is protonated by treating with aqueous HCI (1M). Usually, two nearby imine sites are protonated to form a bipolaron (dication salt).
The bipolaron then undergoes a further rearrangement to form two polarons in which positive charges are delocalized as shown in the reaction scheme.
The resulting emeraldine salt has delocalized +ve charges in the polymeric back bone which are responsible for electrical conductivity.
Positive charges are compensated by anions (chloride ions) of the acid.
Thus polymer as a whole is electrically neutral but contains delocalised positive charges on the polymer structure which conducts an electric current.
This type of protonic acid doping increases the conductivity of polyaniline by 9 to 10 times.
Only 1% of the charge carriers which are available in the Emeraldine salt actually contribute to its observed conductivity.
If all the available charge carriers were to contribute, the resulting conductivity at room temperature would be 10 S/ cm, which is comparable to that of copper.
What is Poly Dimethyl Siloxane?
PDMS (Polydimethylsiloxane) is a very high-performance, transparent, and flexible silicone polymer.
It is a long chain made of repeating smaller units, called monomers.
Chemical repeat unit of PDMS is -[Si(CH3)2O]-n.
The backbone is made of silicon (Si) and oxygen (O) atoms, with two methyl groups (-CH3) attached to each silicon.
Thus, PDMS is a flexible chain of silicon and oxygen, with soft "side arms" of methyl groups.
How is PDMS synthesized?
PDMS is usually synthesized in two main steps:
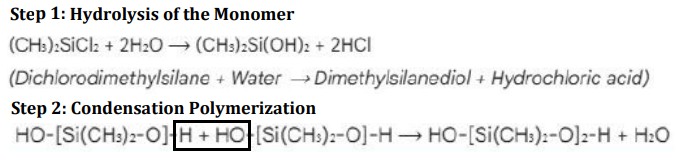
Step 1: Hydrolysis of the Monomer
Monomor: Dichlorodimethylsilane ((CH3)2 SiCl2) is hydrolysed with water, replacing Cl groups with OH groups → Silanol (reactive intermediate)
Step 2: Condensation Polymerization
Silanol molecules don’t stay separate for long.
OH groups of Silanol groups link up by a strong siloxane bond (Si-O-Si) releases a molecule of water
This repeats to form long liquid chains of the polymer
Step 3: Curing (Turning the Liquid into a Solid Rubber)
Linear chains of PDMS is a thick honey-like liquid
treated with cross-linking agents like tetraethyl orthosilicate, TEOS and Pt-based catalysts
Introduces chemical bridges between the long polymer chains
Forming a solid 3D network → strength and elasticity (rubbery)
What are the Properties of PDMS?
It is flexible and elastic because of its Si-O bonds which are stronger and more flexible than C-C bonds in polymers. It can be stretched 100-300%, twisted, and bent without breaking, just like skin.
It is biocompatible. It is non-toxic and FDA-approved for medical use. It doesn't cause reactions with human skin or tissue. This is a must-have for any wearable or implantable device.
It has high optical transparency. It is crystal clear. This is useful for LEDs or optical sensors applications.
It is hydrophobic due to methyl side groups. It naturally repels water, which helps protect electronics from sweat and moisture.
It is permeable to gases. It allows gases like oxygen and carbon dioxide to pass through. Thus it can breathe in e-skin applications.
It is a very good electrical insulator. It does not conduct electricity, making it a perfect base (substrate) on which to build electronic circuits.
It is stable up to 200°C and decomposes above 300°C stable due to Si-O bond energy
Applications in RFID (Radio Frequency Identification)
RFID is a technology used for wireless identification using electromagnetic waves.
RFID tag is a wireless barcode tag with antennas that send info when scanned by radio waves.
These are used for tracking everything from products in a store to people like in metro cards, toll tags, inventory tracking and patient monitoring
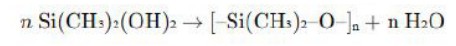
Why is PDMS is used as a base substrate for RFID tags?
By building the RFID antenna on a PDMS substrate, we can create tags that are not flat and rigid but flexible and wearable. These can be bent or even embedded into clothing. It acts as a protective encapsulation layer for fragile RFID circuits.
PDMS layer can hold a stretchable conductive antenna. It can integrate stretchable conductive patterns like Cu, Ag nanowires, graphene, CNTs for RFID antennas.
Because PDMS is biocompatible, an RFID chip can be encapsulated within it and safely implanted under the skin as implantable tag. It is not rejected by body and can be used for medical identification or secure access.
The washable and waterproof nature of PDMS protects the fragile RFID antenna and chip from physical damage and moisture. This makes the tags much more durable for use in harsh environments.
What is PolyVinylidene Fluoride and how is it made??
Semi-crystalline polymer of Vinylidene Fluoride (VDF) monomer with a repeat unit (CH2-CF2)n
It is made by free radical polymerization of VDF using initiator as dibenzoyl peroxide or ammonium persulfate.
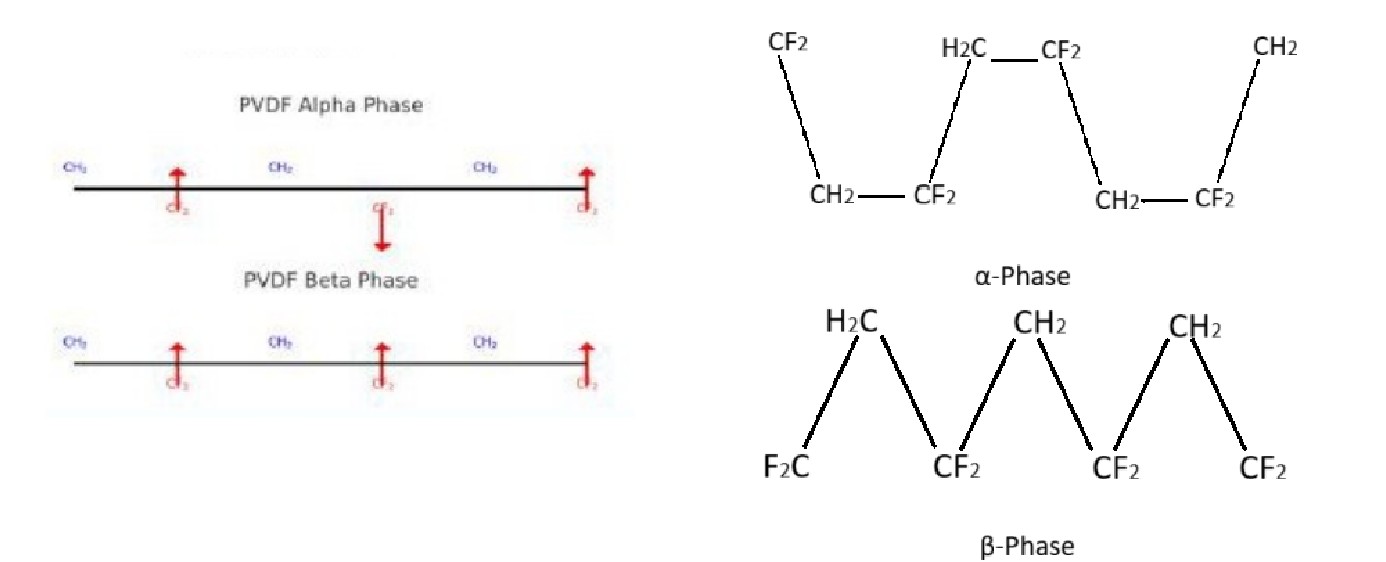
Describe the different crystalline phases of PVDF
α-Phase which is a most common phase. It is non-polar and has twisted chain structure. The dipoles of C-F bonds are arranged in alternating directions and they cancel out. Hence, no net polarization.
β-Phase is highly polar where the dipoles (C-F bonds) are aligned in one direction. Hence, it has strong net polarization. It has dipole moment 2.1 D per unit. It has straight chain structure. This is responsible for unique piezoelectric and pyroelectric properties.
γ- and δ-phases are less common.
How to convert α-phase into β-phase?
α-phase can be converted into β-phase by stretch the film 3-5 times at 80-100°C.
Also by applying strong electric field while heating to 70-100°C. This aligns the dipoles (positive-negative ends). This is called as Poling.
What are some properties of PVDF with β-phase?
Piezoelectric property.
It generates electric charge when mechanically deformed.
If you press, bend, or stretch it, it generates a small voltage.
Conversely, if you apply a voltage to it, it will slightly change its shape (expand or contract).
This happens because in the β phase, the dipoles (C-F bonds) are aligned in one direction.
Pyroelectric property.
It generates a voltage when temperature changes.
This makes it useful for thermal sensors.
High Chemical Resistance:
It is hydrophobic (repels water), resistant to acids, solvents, and other harsh chemicals.
It is thermally stable up to ~170 °C.
It is UV stable and lasts 20+ years outdoors without yellowing.
Strong and Flexible: Tough polymer with high flexibility compared to other fluoropolymers.
Ferroelectric and can hold charge: It has dielectric constant of 8-12 which is good for capacitors.
What is E-Nose?
E-nose is an artificial nose that mimics the human sense of smell by using an array of sensors to detect and identify different gases or odors.
How is PVDF used in E-Nose?
PVDF films are used as the sensing layer because they are piezoelectric.
When gas molecules adsorb on PVDF film, they change mass or strain on the surface.
This causes a measurable change in electrical signal.
They can be coated with nanoparticles, enzymes, or polymers to selectively detect different gases/odors.
Explain the working of PVDF in E-Nose
In an electronic nose, a thin PVDF is coated on an electrode which can adsorb certain gas molecules (the "smell").
The PVDF sensor is made to vibrate at its natural high frequency using its piezoelectric property.
When the target gas molecules land on the coating, they add a tiny amount of mass to the sensor.
This extra mass changes the vibration frequency of the PVDF.
Piezoelectric effect converts this change into electrical signal.
This signal is analysed to identify the odor (like detecting food spoilage, environmental gases, explosives).
By using an array of PVDF sensors with different coatings, the e-nose can identify the unique "frequency fingerprint" of a complex smell.
What are Polymeric Semiconductors and why aren’t they used in practical field?
Semi-conducting polymers are having the energy gap between the valence band and conduction band (band gap) are not so large and not so small. They have low conductivity.
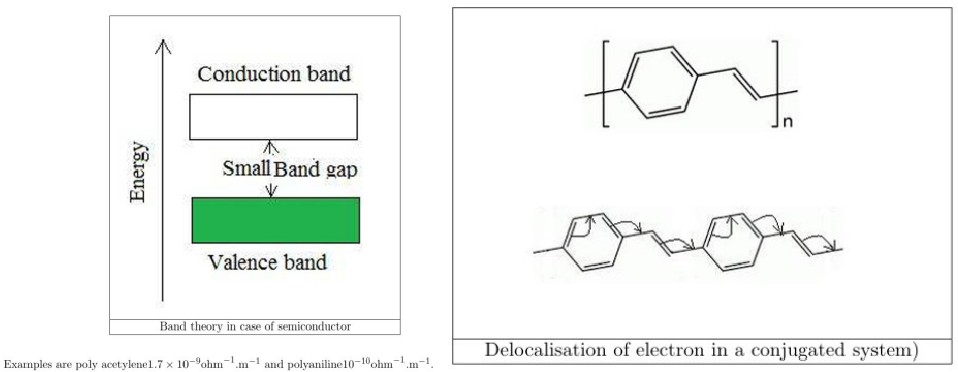
What are the different ways to enhance conductivity of semiconductors?
Doping
Filling polymer with Conducting materials
Blending with Conducting polymer
Explain Doping
The conduction power of semiconductor can be enhanced by input of some foreign material or impurities.
These impurities are called doping agent or dopant.
Appropriate doping agent increase the conductivity of semiconductors by many folds.
The increase in conduction is due to participation of impurity elements in between the valence band and conduction band and thus making a bridge through which electrons can jump easily from the valence band to the conduction band
What are the 2 types of Doping? or Distinguish n-type and p-type polymeric semiconductor materials
P-Type Doping through Oxidation of Materials
In this type of doping some electrons from the conjugated bonds are removed through oxidation, creating a positive hole called polaron inside the polymer.
The positive hole or polaron can move throughout the polymeric chain and make it conducting polymer. Lewis acids (FeCl3,AlCl3) are generally use as doping agent.
N-Type Doping through Reduction of Materials
In this type of doping some electrons are introduced to the conjugated bonds through reduction creating a negative hole or charge inside the polymer.
The negative hole or charge can move throughout the polymeric chain and make it conducting polymer.
Lewis bases (Na+C10H8-,K+C10H8-) are generally use as doping agent.
What properties are required for an ideal structural material (air craft industry)? Does any metal exist with these properties?
Low density
High strength and stiffness
Corrosion resistance
Abrasion and impact resistance.
No single metal, alloy, ceramic or polymeric materials are known that can offer combination of aforesaid properties because a strong material is relatively dense and an increase in stiffness generally results in a decrease in impact strength.
What are Polymer Composites?
A combination of two or more distinct components to form a new class of material suitable for structural applications is referred as composite materials
What are the Components of Polymer composites?
Polymer composites are generally made of two components, namely Matrix and Fibre
The fibre is generally embedded in the matrix in order to make the matrix stronger.
The matrix is usually a thermoset material such as epoxy resin and it holds the fibre together.
Fibre is most often glass; other synthetic fibres include carbon fibre, Kevlar etc. Naturally occurring fibres such as wood - lignin, cellulose can also be employed.
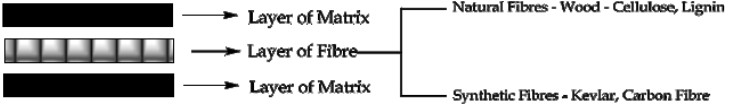
What is the Importance of structure material of Polymer Composites?
Matrix binds the fibers together and can transmit and distribute any externally applied stress to the fibers.
Matrix protects individual fiber from surface damage due to mechanical abrasion or any chemical reaction with the surroundings.
The strength of the composite material is primarily due to the bonding forces between fibers and matrix.
What is Epoxy Resin-Magnetite (Fe3O4) Composite?
Epoxy resin-magnetite (Fe3O4) composites are hybrid materials that combine the mechanical strength and chemical stability of epoxy resins with the magnetic, electrical, and sensing properties of magnetite nanoparticles (Fe3O4).
Such composites have attracted significant interest for sensor applications, electromagnetic shielding, and smart materials due to their tunable electrical conductivity, magnetic response, and dielectric properties.
Epoxy resin is a thermosetting polymer containing epoxide (-C-O-C-) groups that can crosslink with hardeners (like amines).
It has high mechanical strength, good adhesion and chemical resistance, and electrical insulation and thermal stability.
Magnetite (Fe3O4) is a ferrimagnetic iron oxide nanoparticle.
It has strong magnetic moment, electrical conductivity due to Fe2+/Fe3+ ions, high surface area and it is biocompatible.
What is the Ultrasonication Method used in the Synthesis of Epoxy Resin-Fe3O4 Composite?
The ultrasonication method is a simple, efficient route to disperse nanoparticles uniformly within a polymer matrix.
Materials required are:
Epoxy resin (e.g., diglycidyl ether of bisphenol A, DGEBA)
Hardener (e.g., triethylenetetramine, TETA)
Fe3O4 nanoparticles (synthesized or commercially obtained)
Solvent (optional): acetone or ethanol (to aid dispersion).
Explain the steps of Synthesis of Epoxy Resin-Fe3O4 Composite by Ultrasonification Method
Step 1: Preparation of Fe3O4 Nanoparticles (if synthesized in-lab)
Chemical co-precipitation method:
Mix FeCl3.6H2O and FeCl2.4H2O in a 2:1 molar ratio.
Add to NaOH or NH4OH solution under stirring at 60-80°C.
Black precipitate of Fe3O4 forms.
Wash and dry the nanoparticles.
Fe2+ + 2Fe³+ + 8OH- → Fe3O4 + 4H2O
Step 2: Dispersion of Fe3O4 Nanoparticles (Ultrasonication)
Add a specific wt% of Fe3O4 nanoparticles (e.g., 1-10%) into epoxy resin.
Use an ultrasonic probe or bath (20-40 kHz) for 30-60 mins to achieve uniform dispersion and prevent agglomeration.
The high frequency ultrasound waves create cavitation, which breaks particle clusters and distributes them evenly in the resin.
Step 3: Mixing with Hardener and Curing
Add the hardener to the Fe3O4-epoxy mixture in the proper ratio (usually 10:1 or as recommended).
Stir gently to avoid air bubbles.
Pour into molds
Cure at room temperature or elevated temperature (60- 80°C) for several hours.
Step 4: Post-Curing and Finishing:
After curing, remove the samples
Post-cure (if required) to ensure full crosslinking.
The resulting composite is solid, smooth, and magnetically active.
What are the Properties of Epoxy Resin-Fe3O4 Nanocomposite?
The composite exhibits synergistic properties from both the polymer matrix and the magnetic nanoparticles.
Structural and Morphological Properties:
Interfacial bonding between Fe3O4 and epoxy enhances mechanical strength.
Nanoparticle size (typically 10-50 nm) influences electrical and magnetic performance.
Mechanical Properties:
Increased hardness and tensile strength with optimal nanoparticle loading.
Improved toughness due to good stress transfer between Fe3O4 and epoxy.
Electrical and Dielectric Properties:
Addition of Fe3O4 increases electrical conductivity and dielectric constant.
The composite transitions from insulating to semiconducting behavior.
Dielectric loss increases slightly due to interfacial polarization (Maxwell-Wagner effect)
Magnetic Properties:
The composite becomes magnetically responsive (superparamagnetic or ferrimagnetic).
Magnetic saturation decreases slightly compared to pure Fe3O4 due to the polymer matrix.
Useful for magnetic field sensing and electromagnetic shielding.
Thermal Properties:
Enhanced thermal stability and glass transition temperature (Tg).
Fe3O4 nanoparticles act as heat dissipation centers.
Explain Sensor Applications of Epoxy-Fe3O4 Nanocomposite
Magnetic Field Sensors: Detects changes in magnetic field via variations in resistivity or inductance. Useful for non-contact sensing and position detection.
Pressure and Strain Sensors: Electrical resistance of the composite changes under deformation. Suitable for flexible or structural health monitoring sensors.
Temperature Sensors: Resistance varies with temperature due to magnetoresistive effects. Hence, can be used in temperature sensor.
Gas and Chemical Sensors: Surface-modified Fe3O4-epoxy composites show selective gas adsorption leading to resistance change. Hence, it can be used in Gas and Chemical Sensors
What are Kevlar Fiber Reinforced Polymer (KFRP) composites?
Kevlar Fiber Reinforced Polymer (KFRP) composites are advanced materials where high-strength Kevlar (aramid) fibers are embedded in a polymer matrix, like epoxy or polypropylene.
They are lightweight, durable structures with exceptional mechanical and thermal properties.
Kevlar is renowned for its five times the strength of steel at one-fifth the weight.
It makes KFRP ideal for demanding applications.
In smart electronic devices such as wearables, flexible sensors, and structural supercapacitors, these composites enable multifunctional designs that combine mechanical robustness with electrical functionality, supporting trends toward flexible and sustainable electronics.
Explain the Synthesis of KFRP Composites
Synthesis involves combining Kevlar fibers with a polymer matrix through processes that ensure strong fiber-matrix bonding for load transfer.
The goal is uniform dispersion and minimal voids, achieved via chemical treatments or advanced fabrication.
Key steps and methods:
Kevlar Fiber Preparation: Kevlar fibers (Kevlar 29 or 49, diameter 10-20 µm) are used fibers.
Matrix Selection and Mixing: Thermoset matrices (e.g., epoxy from bisphenol A + epichlorohydrin) or thermoplastics (e.g., polypropylene) are used.
Fabrication Techniques:
Additive Manufacturing: This technique is used for obtaining composites suitable for electronics. It prints continuous Kevlar-reinforced filaments at 220-250°C nozzle temperature. Achieves anisotropic strength upto 200 MPa along fibers.
What are the Properties of KFRP Composites?
Mechanical Properties: It exhibits exceptional tensile strength (200-500 MPa for composite)
Higher Young's modulus 70-130 GPa.
Thermal Properties: It is stable in the temperature range -196 to +204°C. Low thermal conductivity for insulation.
Electrical Properties: It has low dielectric constant (3-4), making it suitable for insulating flexible circuits. Kevlar's conductivity is near-zero, but doping with CNTs raises it to 10-3 S/cm for antistatic uses.
It's density is 1.4-1.6 g/cm³. It is a lightweight polymer. It has high chemical resistance to oils and acids).
Explain the Application of KFRP in Smart Electronic Devices
KFRP is used in the following smart electronics applications due to its flexibility, strength, and low weight:
Wearable Electronics: It is used in flexible sensors in smartwatches/fitness trackers. It provides bendable, impact-resistant housing; elongation >3% prevents cracks during motion.
Structural Supercapacitors: It is used as energy-storing composites for EVs/drones. It acts as load-bearing electrode due high 200 MPa strength.
Flexible PCBs/Antennas: It is used in bendable circuit boards for IoT devices. It is insulating substrate with EMI shielding and thermal stability for high- speed signals.
Portable Chargers/Smart Textiles: It is used in solar-integrated fabrics or foldable batteries. It's lightweight reinforcement and vibration damping extends device life in mobiles.
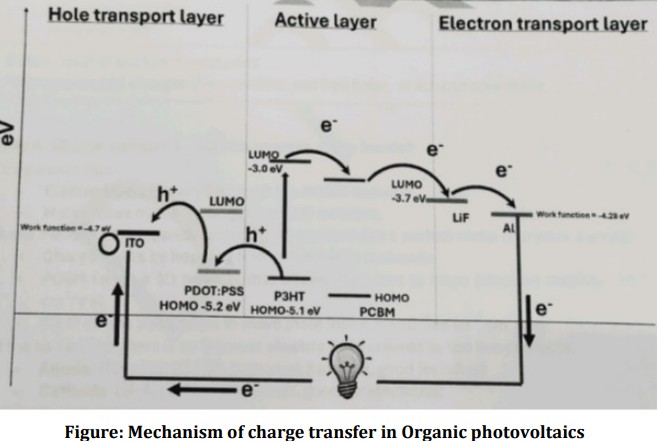
Explain the Construction of Organic Photovoltaics
(Poly(3-hexylthiophene) (P3HT) as a donor and phenyl C61-butyric acid methyl ester (PCBM) as an acceptor)
Glass substrate : Just supporter like a plate.
Indium Tin Oxide (ITO): Anode Transparent conducting electrode- collects holes.
PEDOT: PSS : [Hole transport layer] Blocks electron and allow holes to ITO
Active layer : [P3HT: PCBM blend] Light absorbed Exciton Interface
LiF : [Electron Transport layer] Block holes, pushes electron to Aluminium
Aluminium cathode : Metal that collects electrons
![<ol><li><p><span style="color: red;">(Poly(3-hexylthiophene)</span> (P3HT) as a <span style="color: red;">donor</span> and <span style="color: red;">phenyl C61-butyric acid methyl ester</span> (PCBM) as an <span style="color: red;">acceptor</span>)</p></li><li><p>Glass substrate : Just supporter like a plate.</p></li><li><p>Indium Tin Oxide (ITO): Anode Transparent conducting electrode- <span style="color: red;">collects holes. </span></p></li><li><p>PEDOT: PSS : <span style="color: red;">[Hole transport layer]</span> Blocks electron and allow holes to ITO</p></li><li><p>Active layer : [P3HT: PCBM blend] <span style="color: red;">Light absorbed Exciton Interface</span></p></li><li><p>LiF : <span style="color: red;">[Electron Transport layer]</span> Block holes, pushes electron to Aluminium</p></li><li><p>Aluminium cathode : Metal that <span style="color: red;">collects electrons</span></p></li></ol><p></p>](https://knowt-user-attachments.s3.amazonaws.com/874a8ff8-e4bc-4870-9324-b295f8a195c7.jpg)
Explain the Working of Organic Photovoltaics
When light illuminates, it enters from the glass/ITO side and reaches the P3HT:PCBM active layer. After that there are four steps through which it produces voltage.
Step 1: Photon Absorption:
P3HT absorbs light between 400 to 650 nm wavelength radiation.
As a result, an electron jumps from HOMO (-5.1 eV) to LUMO (-3.0 eV) and leave behind positively charged “hole” in the HOMO.
Step 2: Exciton diffusion:
The excited electron has life time of 100 picoseconds to 1 nanosecond, so the electron immediately moves from P3HT LUMO to PCBM LUMO.
Step 3: Exciton dissociation:
at the same time the holes move into HOMO of PDOT:PSS.
Now the electron and hole no longer in the same place.
Step 4: Charge Transport:
Electrons move mainly through PCBM network and collects these electron at aluminum cathode electrode.
Whereas holes move through the P3HT network and collects at the anode electrode.
These anode and cathode electrodes are connected to voltameter/devices to measure the voltage produced.
➢ Holes walk through P3HT ⇾ PEDOT:PSS ⇾ ITO layer (Anode)
➢ Electrons walk through P3HT ⇾PCBM ⇾ LiF ⇾ Al (Cathode)
List some Applications of Organic Photovoltaics
Organic Solar Cells (OSCs): The primary application is in solar cells, particularly for small, flexible, or transparent applications where silicon-based cells are not suitable.
Flexible Photovoltaics: The ability to be processed from solution makes them suitable for flexible and lightweight solar cells.
Building-Integrated Photovoltaics (BIPV): Potential for integration into windows or building facades due to transparency possibilities.
Portable Electronics: Powering portable electronic devices where lightweight and flexible energy sources are needed.
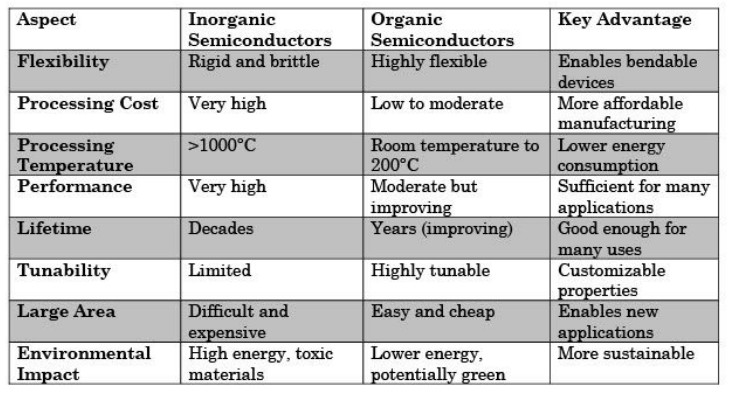
Distinguish between Organic and Inorganic semiconductors