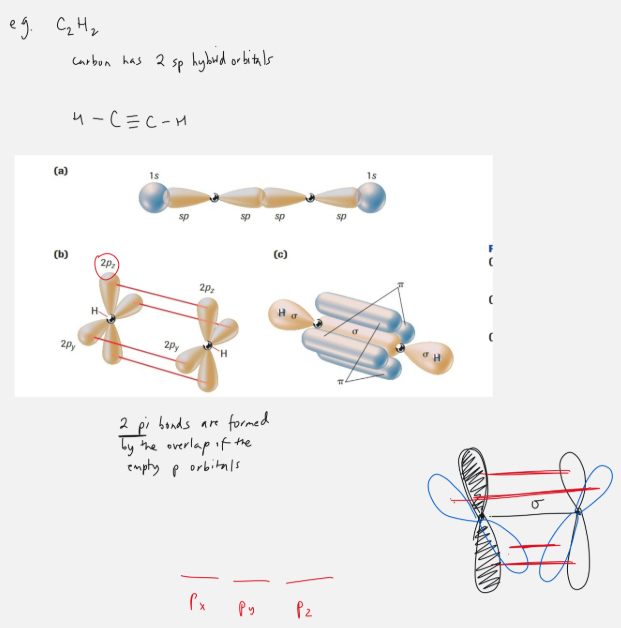
hybrid orbitals
1/5
Earn XP
Description and Tags
26/7/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
what is a hybrid orbitals
two or more orbitals merge together to form a hybrid orbital
sp3 orbital
ex. carbon
special stability occurs: one s orbital electron gets promoted to empty p orbital
2s and 2p orbital hybridize to form intermediate sp3 orbital
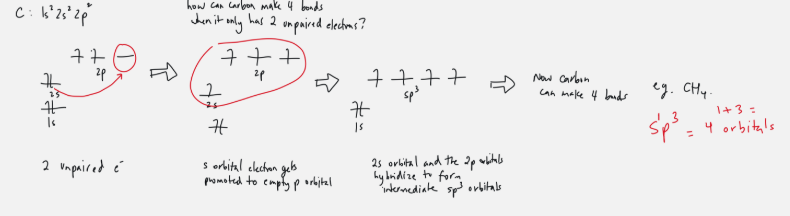
Carbon can make 4 bonds
sp3: 1 s orbital + 3 p orbitals = 4 orbitals to form bonds with
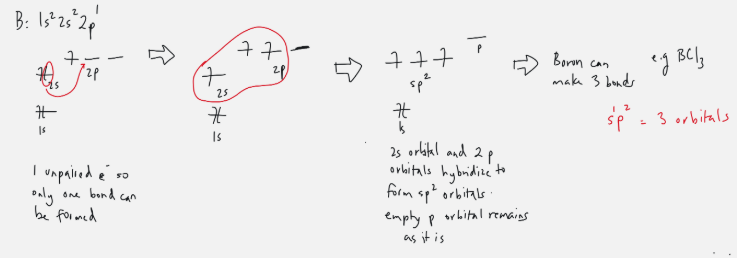
sp2 orbital
ex. Boron
one s orbital electron gets promoted to 2p orbital, three are now filled
two 2p orbital + 2s orbital merges to form sp2
sp2: 1 s + 2 p = 3 orbitals to form bonds with
one p orbital is left empty
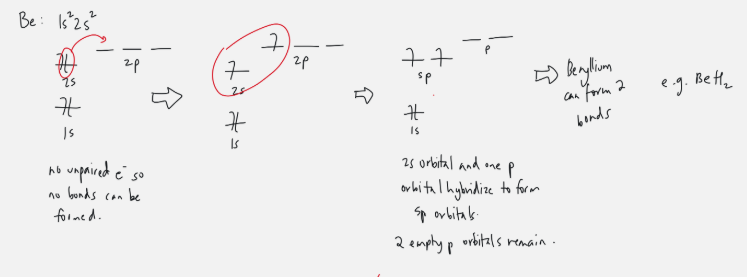
sp orbital
ex. Beryllium
one s orbital electron gets promoted to empty 2p orbitals
2s and one 2p orbital hybridize to form sp
two p orbitals left empty
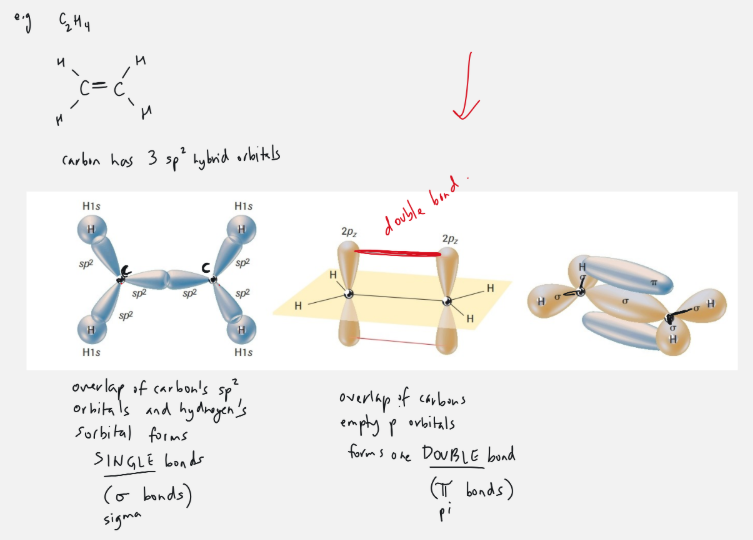
double bond
ex. carbon
in C2H4 carbon has 3 sp2 orbitals
triple bond
ex. carbon
in C2H2 has 2 sp orbitals