Microbiology
1/303
Earn XP
Description and Tags
Chap 8,9,11,12
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
304 Terms
Metabolism
Change; all chemical reactions and physical workings of cell
Anabolism
(Biosynthesis) synthesis of cell molecules and structures; building and bond - making process that forms macromolecules and requires energy
Catabolism
Breaks bonds of larger molecules into smaller ones; releases energy
Types of energy reactions
Exergonic and endergonic
Exergonic
Reactions release energy and stored in high energy phosphate bonds
Endergonic
Reactions require energy
Where does energy come from
Light and chemical bonds
When is energy stoved
ATP
What do cells require
Constant input and expenditure of usable energy
What drives cell transactions
Chemical energy
Classifications of organisms by energy and carbon source
Chemotrophs and phototrophs
Chemotrophs
Chemocetotrophs and chemoheterotrophs
Chemoautotroph'S
Energy source is chemical, carbon source is inorganic, examples are hydrogen, sulfur, and iron
Chemoheterotrophs
Energy source is chemical, carbon source is organic, examples are all animals, most fungi, and Protozoa
Phototrophs
Photoautotrophs and photoheterotrophs
Photoautotrophs
Energy source is light, carbon source is inorganic, examples are all plants, algae, and Cyanobacteria
Photoheterotrophs
Energy source is light, carbon source is organic, examples are green and purple nonsulfur bacteria
Metabolic pathways
Can be catabolic or anabolic, each reaction is catalyzed by its own enzyme
Pathway types
Linear, branched, cyclic
Enzymes
Proteins that accelerate rate of reaction without being changed themselves, lower activation energy, provide a way to control or regulate biochemical reactions, enzymes won't occur unless enzyme that catalyzes reaction is present and active
Sucrose
Glucose and fructose
What are biochemical reactions controlled by
Changes in enzyme activity
Changes in amount of enzyme or substrate
More enzyme and or more substrate equals more product
Change in temp, ph, or salt
Affects enzyme structure
Availability of necessary cofactors
Some enzymes don't work without a non-protein cofactor
Effect of inhibitors
Molecules that bind enzymes and reduce their activity
Ph
Enzyme structure depends on ph, it affects charge of r groups
Temperature
Reactions occur more rapidly as temperature rises, as long as enzyme is active
Substrate
Reactions occur more rapidly as substrate rises, saturation occurs when substrate is high enough
Enzyme Denaturation
Enzymes are polypeptides that retain their ability to function only when folded properly, precise 3D structure
What causes protein to unfold
Change in temp, ph, or salt concentration can disrupt amino acid R group interactions
What is it called when proteins unfold
Denatured; interactions are disulfide bridges, ionic bonds, and hydrophobic interactions
cofactors
inorganic ions (Fe2+)
Coenzymes
organic molecules, dietary vitamins
apoenzyme
becomes active by binding of coenzyme or cofactor to enzyme
holoenzyme
formed when associated cofactor or coenzyme binds to enzymes active state
inhibitors bind enzymes in two ways
competitive and allosteric
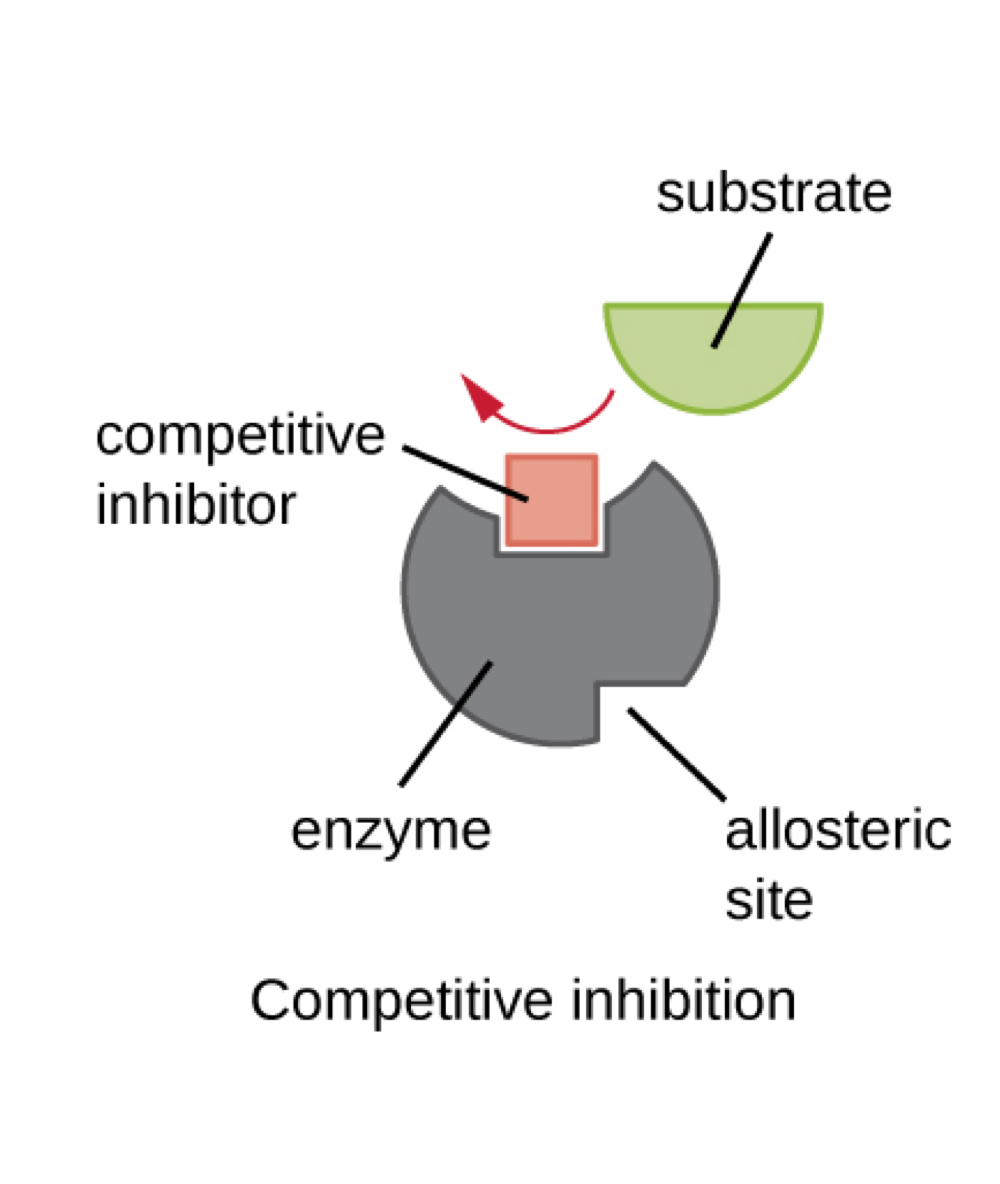
competitive inhibition
binding to active site
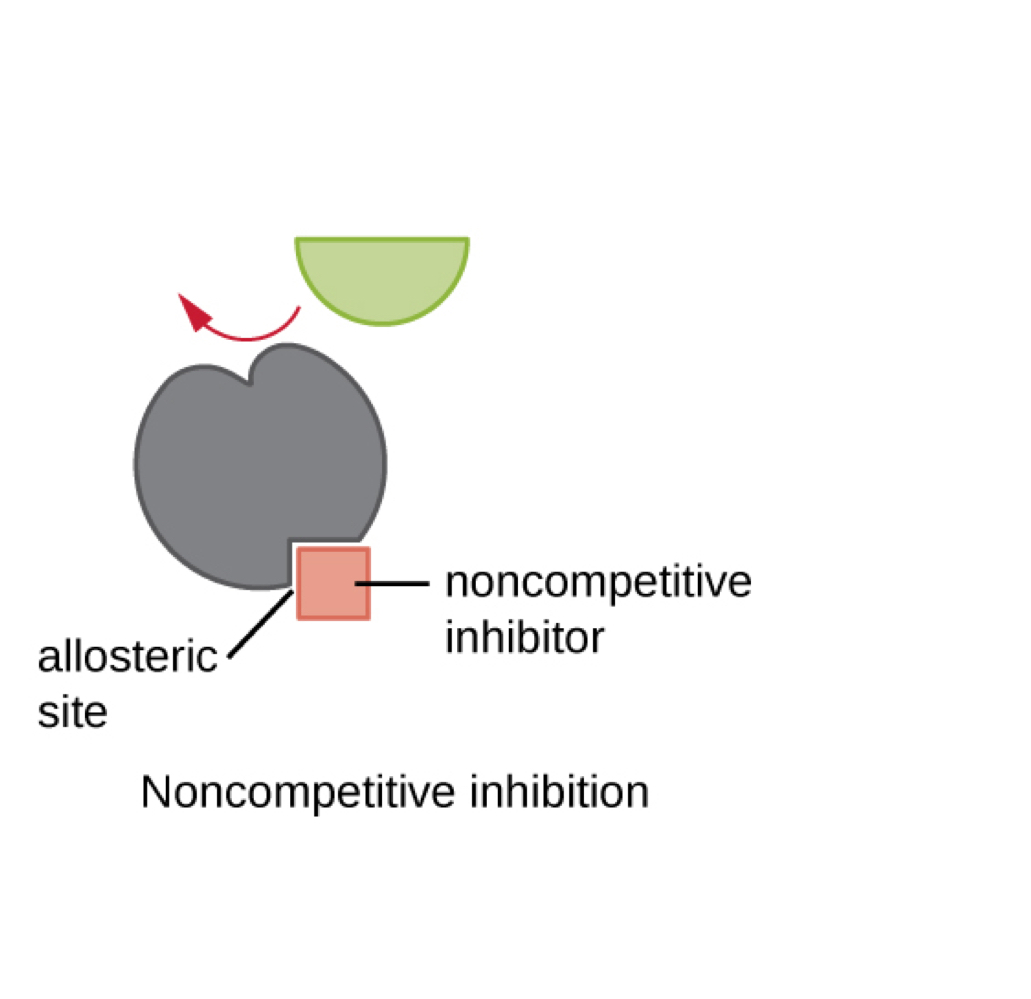
allosteric inhibition
binding elsewhere, changing shape
how do inhibitors bind
reversibly or irreversibly
feedback inhibition
end-products of metabolic pathways are important reversible enzyme inhibitors
feedback inhibition
inhibit first enzyme in pathway, turning pathway off
low inhibitor
pathway ON
high inhibitor
pathway OFF
ATP
adenosine triphosphate (ATP)
what is atp
source of useable energy of ALL cells
what is food energy converted to
atp
what exactly is atp
breaking bond of third phosphate releases ideal amount of energy (bond is easily broken)
how is atp produced
glycolysis followed by either fermentation (Low atp yield) or respiration (high atp yield)
Glycolysis
catabolic pathway where sugars are broken down to two 3-carbon molecules of pyruvic acid (or pyruvate)
how many atp come from glycolysis
2 atp per glucose; also transfers high energy electrons to NAD+ to yield 2 NADH
OIL/RIG
? idk it says to remember this
how is energy in food molecules captured
as high energy electrons by electron carriers (cofactors) such as NADH and FADH2
Reduction
when a molecule receives/gains electrons (e- are usually transfered as part of hydrogen atom)
oxidation
a molecule that loses electrons (loses H+)
fermentation
atp production begins and ends with glycolysis in organisms that ferment
what is recycled during fermentation
NAD+ so that glycolysis can continue
why does glycolysis need NAD+
to pick up high energy electrons from glucose splitting
how is NADH oxidized to NAD+
reducing pyruvate to lactic acid (For example)
what does fermentation result in
reduction of pyruvate to form lactic acid
fermentation process
lactic acid gains electrons (RIG) generated from glycolysis carried by NADH; NADH is oxidized (OIL) to form NAD+; NAD+ is replenished and glycolysis continues
what are used to regenerate NAD+ from NADH
organic molecules
when did fermentation evolve
long before aerobic respiration when little oxygen was present in the atomosphere
respiration
energy in pyruvate and NADH are used to produce more ATP
krebs cycle
breaks down pyruvate to 3 CO2, energy captured as electrons by NADH and FADH2
electron transport
electrons from NADH and FADH2 are used to produce a H+ gradient
chemiosmosis
H+ gradient used to make atp
Krebs Cycle
cyclical metabolic pathway catalyzed by enzymes in the matrix of mitochondria or cytoplasm of bacteria; pyruvate is converted to Acetyl-CoA which enters the cycle; generates 2 ATP; generates a lot of NADH/FADH2; energy stored in pyruvate is converted to NADH/FADH2
what is pyruvate converted to in krebs cycle
Acetyl-CoA
how many atp come from kreb cycle
2 atp
what is more importantly generated by the krebs cycle
a lot of NADH and FADH2
what are NADH and FADH2
high energy electron carriers
what is energy stored in pyruvate converted to
NADH and FADH2
how many nadh and fadh2 does krebs produce
6 NADH and 2 FADH2
how many nadh does pyruvate converted to Acetyl-Coa make
2 NADH
how many NADH does glycolysis produce
2 NADH
where do bacteria complete electron transport chain
in the cell
oxidation does what
loses electrons
reduction does what
gains electrons
why use fermentation
some bacteria live in anoxic environments and can not use oxygen; they never evolved the ability to respire
aerobic respiration
Pseudomonas aeruginosa; final electron acceptor is O2; max yield of atp molecules is 38
anaerobic respiration
paracoccus denitrificans; final e- acceptor are NO3-, SO4-2, FE+3; max yield of atp molecules is 5-36
fermentation
candida albicans; final e- acceptor is organics (pyruvate); max yield of atp molecules is 2
what else can be used to produce atp energy
lipids and proteins
proteases
different amino acids enter krebs cycle or glycolysis at various stages
lipases
fatty acids are broken down to acetyl groups and fed in krebs cycle
autotrophic processes
carbon dioxide plus water with suns energy and chlorophyll produce sugars and oxygen
autotrophs
make their own food and can produce organic molecules from CO2 (an inorganic carbon source)
heterotrophs
require an organic source of carbon
what come from autotrophs
organic molecules, directly or indirectly
source of energy for autotrophic processes
light or chemical
light source for autotrophic processes
photoautotrophs that carry out photosynthesis
chemical source for autotrophic processes
chemoautotrophs that use various molecules as a source of high energy electrons
what phases does photosynthesis have
light and dark
what do atp and NADH provide in the dark reactions
energy to fuel production of sugars
electrons (from H2O) energized by sunlight
fuel synthesis of atp through electron transport and chemiosmosis; reduce NADH to NADPH
dark reactions of photosynthesis
involves anabolic pathway which is Calvin-Benson cycle
Calvin-Benson
endergonic reactions are fueled by atp and nadph from “light” reactions; process of carbon fixation (converting CO2 to organic compounds); sugars can be used as energy or to build other organic molecules
carbon fixation
converting CO2 to organic compounds
how do bacteria divide
binary fission