electronegativity and polarity
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
what is electronegativity
how strongly an atom can pull the bonded pair of electrons towards themselves
what is the most electronegative element
fluorine
how is electronegativity measured
on the Pauling scale
how does electronegativity increase on the periodic table
as you go up and right
are they ionic or covenant bonds
if they are the same element they are covenant
if they have a small difference in electronegativity they will be polar covalent
if they have a big diff in electronegativity (above 1.8) they are ionic
where is the electron pair in a non polar bond
in then middle
where is the electron pair in a polar bond
nearer the more electronegative atom
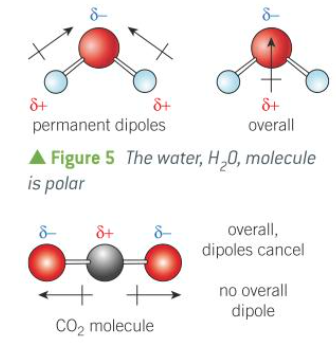
what does it mean if a bond if a bond is polar
one side of the molecule will have a partial positive charge while the other will have a partial negative charge (a dipole)
if a dipole doesn’t change it is called what
a permanent dipole
when do dipoles form (what shapes)
when the molecule doesn’t have a symmetrical shape
what happens to polar solvents in water
they dissolve