General Chemistry Master Set
1/168
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
169 Terms
element
cannot be broken down chemically into simpler substance
compound
two or more elements chemically joined in fixed ratio
has different properties from elements it formed from
mixture
two or more elements not chemically bonded together
retains properties from elements it formed from
solid to gas
sublimation
gas to solid
deposition
solid
has fixed shape and volume
liquid
has fixed volume but no fixed shape
gas
has neither fixed shape or volume
physical change
no new substances are produced
melting of ice is an example
chemical change
results in the formation of new chemical substances
atomic structure
protons and neutrons (nucleons) are located in nucleus of atom
electrons are located in energy levels surrounding nucleus
principal energy level (n)
electrons are located in principal energy levels
the first energy level (n=1) has lowest energy, and energy increases as value of n increases
each main energy level can hold at most 2n² electrons
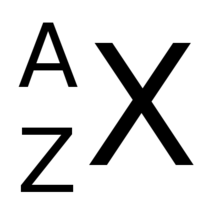
the atomic number (Z) is the number of protons in an atom
the mass number (A) is the number of protons and neutrons (nucleons) in an atom
atoms have same number of protons as electrons
positive ion
less electron than proton
negative ion
more electron that proton
isotope
elements that have same atomic number but different mass number
as isotope mass increases, boiling point, melting point, and density increases
relative abundance
percentage of atoms with a specific mass number in naturally occurring environment
relative atomic mass
mean of the atomic mass of each isotope weighted by relative intensities
only use relative intensities for singular element or diatomic element (but not both)
electromagnetic spectrum
when there is short wavelength, there is high frequency and energy
when there is long wavelength, there is low frequency and energy
violet, indigo, blue, green, yellow, orange, red are in order of shortest to longest wavelength
continuous spectrum
shows all wavelengths of visible light
absorption line spectrum
black lines on coloured background
emission line spectrum
coloured lines on black background
spectral lines converge at higher energy or shorter wavelength
spectroscope
splits light into different wavelengths
bohr model
when electrons absorb photon of energy they transition to higher energy level
when electrons transition to lower energy levels they emit photons of energy
energy of photon emitted for electron transition
E=hf
f is frequency of emission spectrum created by photon
complete emission spectrum
electron transitions to n=3 emit infrared radiation
electron transitions to n=2 emit visible light
electron transitions to n=1 emit ultraviolet light
atomic orbitals are regions where there is a high probability of finding an electron
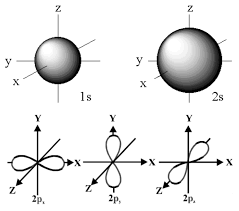
top is s orbitals
bottom is p orbitals
sublevels
each principal energy level is split up into sublevels
n=1 has 1 sub level (1s)
n=2 has 2 sub levels (2s, 2p)
n=3 has 3 sub levels (3s, 3p, 3d)
n=4 has 4 sub levels (4s, 4p, 4d, 4f)
within a principal energy level the order of energy is s<p<d<f
s, p, d, f hold 2, 6, 10, 14 electrons respectively and have 1, 3, 5, 7 orbitals respectively
aufbau principle
lowest energy sublevels are filled first
pauli exclusion principle
atomic orbital must have maximum of two electrons and have opposite spins
hund’s rule
orbitals in a sublevel are filled with one electron each with same spin before being doubly filled
positive ion configuration
lose electrons from highest principal energy level
negative ion configuration
add electrons based on aufbau principle
ions
have same electronic configuration as noble gases
convergence limit
the principal energy levels converge at higher energy
the spectral lines also converge at higher energy
ionisation energy
energy required for the electron transition from n=1 to n=infinity
use E=hf, where f is frequency of convergence limit to get ionisation energy of 1 atom. multiply that by avogadro’s number to get energy per mol.
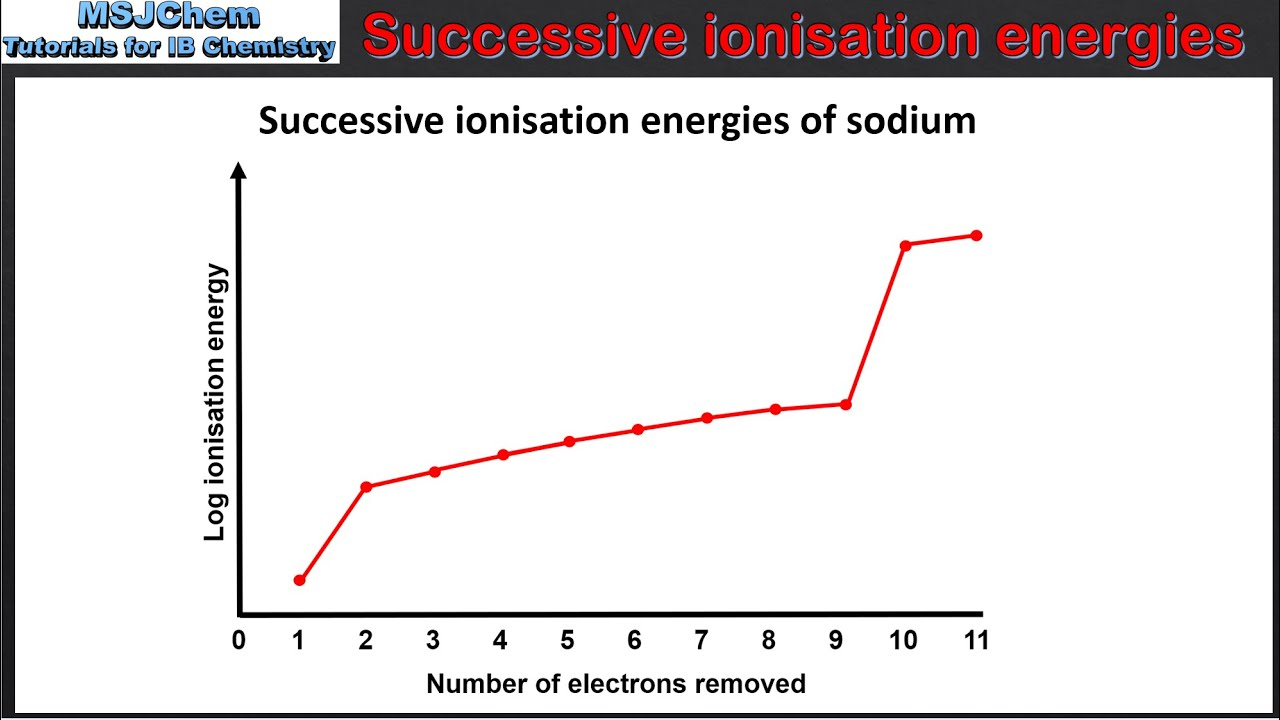
trend in successive ionisation energy
ionisation energy increases as more electrons are removed from gaseous atom due to greater attraction from more positive ion
large increases in ionisation energy when maximum principal energy level changes
ideal gas assumptions
particles in ideal gas are in constant, random, straight line motion
collisions between particles of an ideal gas are elastic
the volume occupied by the particles of the gas are negligible
there are no intermolecular forces between the particles of an ideal gas
when is gas closest to ideal
gases are most ideal at high temperatures and low pressures
they deviate the most at low temperatures and high pressures
cation
positive ion
anion
negative ion
polyatomics
sulfate: SO4 2-
sulfate: SO3 2-
phosphate: PO3 3-
nitrate: NO3 -
nitrite: NO2 -
carbonate: CO3 2-
ammonium: NH4 +
hydroxide: OH -
atoms in polyatomic ion are covalently bonded
bonds in compound that contains polyatomic are ionic
ionic compound properties
have a lattice structure
solids under standard conditions
high melting and boiling points
not conductive when solid, but conductive when molten or dissolved
soluble on polar solvents (like water) where compound is split up into ions
ions are surrounded by water molecules (hydration)
factors impacting melting point of ionic compound
greater the charges on the ions, the higher the melting point
smaller the ionic radius the higher the melting point
lattice enthalpy
energy change when one mol of solid ionic compound is broken down into gaseous ions
endothermic process with a positive enthalpy change
factors effecting lattice enthalpy
greater the charges on the ions, the higher the lattice enthalpy
smaller the ionic radius the higher the lattice enthalpy
nobel gases
group 18
octet rule
atoms bond together to achieve full valence shell of 8 electrons
nobel gases have full valence shells so they are very stable
ionic bonding
electrostatic attraction between oppositely charged ions
always forms between metal (cation) and nonmetal (anion)
transfer electrons to achieve full octet
covalent bonding
share electrons to achieve full octet
occurs between two non metal elements
octet rule exceptions
hydrogen, beryllium, and boron are stable with less than 8 valence electrons
period 3 elements are stable with more than 8 valence electrons (expanded octet)
multiple covalent bonds
in single, double, and triple covalent bond there are 2, 4, 6 shared electrons respectively
bond energy increases and bond length decreases with number of covalent bonds
coordinate covalent bond
one atom contributes both electrons in a bond
VSEPR theory
bonds repel each other to be as far as possible
lone pairs repel each other to be as far as possible
electron domain
can be lone pair, or single/double/triple bond
molecular shapes

electronegativity difference bonding
0-0.4 non polar covalent
0.5-1.7 polar covalent
>= 1.8 ionic
bond dipole
partial negative charge on atoms with high electronegativity
this is due to more electronegative atom pulling shared electrons closer
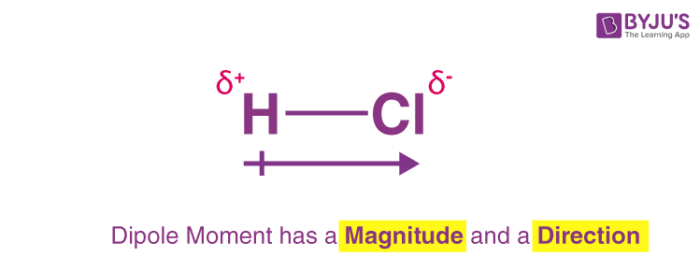
arrow points towards more electronegative atom
non polar molecules
linear, trigonal planar, and tetrahedral molecules with same atoms bonded to the central atom
diatomic molecules with same atoms bonded
molecules with very weak polar bonds (C-H)
polar molecules
linear, trigonal planar, and tetrahedral molecules with different atoms bonded to the central atom
diatomic molecules with different atoms bonded
v-shaped molecules with polar bonds
molecular covalent
covalent substances that exist as individual molecules
intermolecular forces between those molecules
low melting and boiling points due to weak intermolecular forces
polar molecules are soluble in polar substances, nonpolar molecules are soluble in nonpolar substances
nonconductive
giant covalent
covalent bonds that form a lattice structure
there are no intermolecular forces
high melting and boiling points due to strong covalent bonds
insoluble and nonconductive
allotrope
different forms of the same element
graphite allotrope of carbon
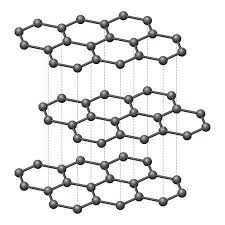
trigonal planar geometry
weak intermolecular forces between layers so layers can slide over each other
good conductor because of delocalised electrons
diamond allotrope of carbon
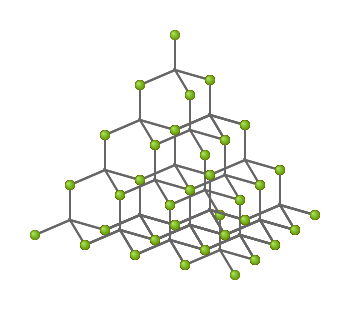
giant covalent structure
high melting and boiling point
tetrahedral geometry
nonconductive
fullerene C60 allotrope of carbon
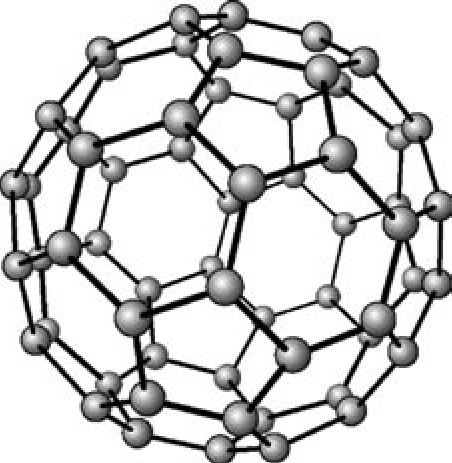
12 pentagons and 20 hexagons
trigonal planar geometry
some conductivity but not as much as graphene
graphene
one layer of graphite (very thin)
good electrical and thermal conductivity
LDF forces
instantaneous dipole formed by random movement of electrons
as molar mass increases strength of LDF increases, which results in higher boiling point
dipole-dipole forces
dipole-dipole forces occur between two polar molecules between partial positive portion of one molecule and partial negative portion of another
stronger the dipole moment the higher the boiling point
hydrogen bonding
very strong dipole-dipole forces caused by hydrogen and F/O/N bond
hydrogen bonding is reason for water’s high boiling point compared to other liquids
strength order of forces
LDF < dipole-dipole < hydrogen bonding < covalent bonding < ionic bonding
factors affecting solubility
polar molecules are soluble in polar substances, nonpolar molecules are soluble in nonpolar substances
most ionic compounds are soluble in water due to strong polar nature causing ion-dipole forces
factors affecting conductivity
covalent substances that exist as molecules have poor conductivity because their electrons are localised to be near to molecule
giant covalent structures also have poor conductivity because their electrons are localised in covalent bonds
when ionic compounds are melted or dissolved their ions are free to move and conduct electricity
metallic substances are good conductors because there is sea of delocalised electrons that are free to move
thin layer chromatography
used to seperate substances based on their solubility in water
rf value is distance substance travels over distance travelled by solvent
more soluble substances have higher rf values
these can be compared to determine what substances were separated
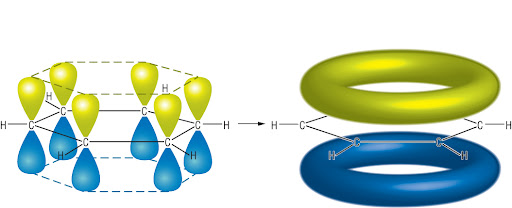
sigma bond
formed along internuclear axis
between two s orbitals/s and p orbital/2 p orbitals
pi bond
formed over internuclear axis
formed between two p orbitals
sigma and pi bonding in covalent
single covalent: 1 sigma bond
double covalent: 1 sigma and 1 pi bond
triple covalent: 1 sigma and 2 pi bond
delocalised pi electrons
exist in all molecules with for which there are resonance structures
electrons are free to move across ions as the orbitals combined
hybridisation
mixing of orbitals to produce hybrid orbitals used for covalent bonding
sp3 hybridisation has 4 orbitals (one s and three p), occurs with 4 electron domains
sp2 hybridisation has 3 orbitals (one s and two p) and leaves 1 unhybridised p orbital, occurs with 3 electron domains
sp hybridisation has 2 orbitals (one s and one p) and leaves 2 unhybridised p orbital, occurs with 2 electron domains
resonance structure
occurs when double bond can be in multiple places in compound
the actual bonds are intermediate between single and double bond
formal charge
used to determine preferred lewis structure of compound
preferred lewis structure has near 0 formal charge for all atoms in compound, negative charges on electronegative atoms
FC = V (valence) - N (non bonding) - B (bonding) / 2
metallic bonding
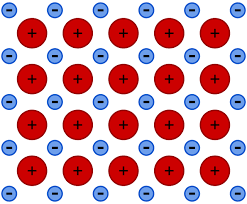
electrostatic force between positive lattice and sea of delocalised electrons
factors effecting metallic bonding strength and melting point
strength of metallic bond increases with greater ionic charge and smaller ionic radius
this also results in a higher melting point
properties of metallic structures
have nondirectional bonds
good conductors of heat and electricity due to presence of delocalised electrons
malleable (can be bent into shape because of layers easily shifting)
ductile (can be drawn into wires)
alloy
alloys are materials that are composed of two or more metals
properties of alloys
alloys are less malleable (harder) because different size atoms make it harder for layers to shift
position of metals, nonmetals, and metalloids on periodic table
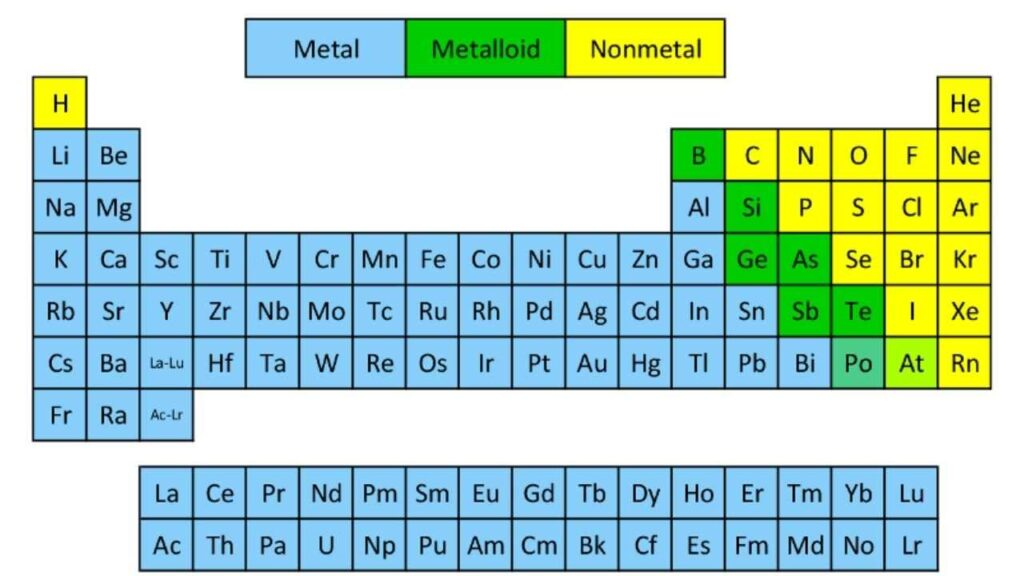
properties of metalloids
have properties of both metals and nonmetals
solids at room temperature
some are shiny
brittle
intermediate electrical conductivity
moderate density
properties of nonmetals
gases at room temperature
dull
brittle
poor electrical conductivity
low density
high ionisation energy
high electronegativity
properties of metals
solids at room temperature
shiny
malleable and ductile
high electrical conductivity
high density
low ionisation energy
low electronegativity
elemental blocks
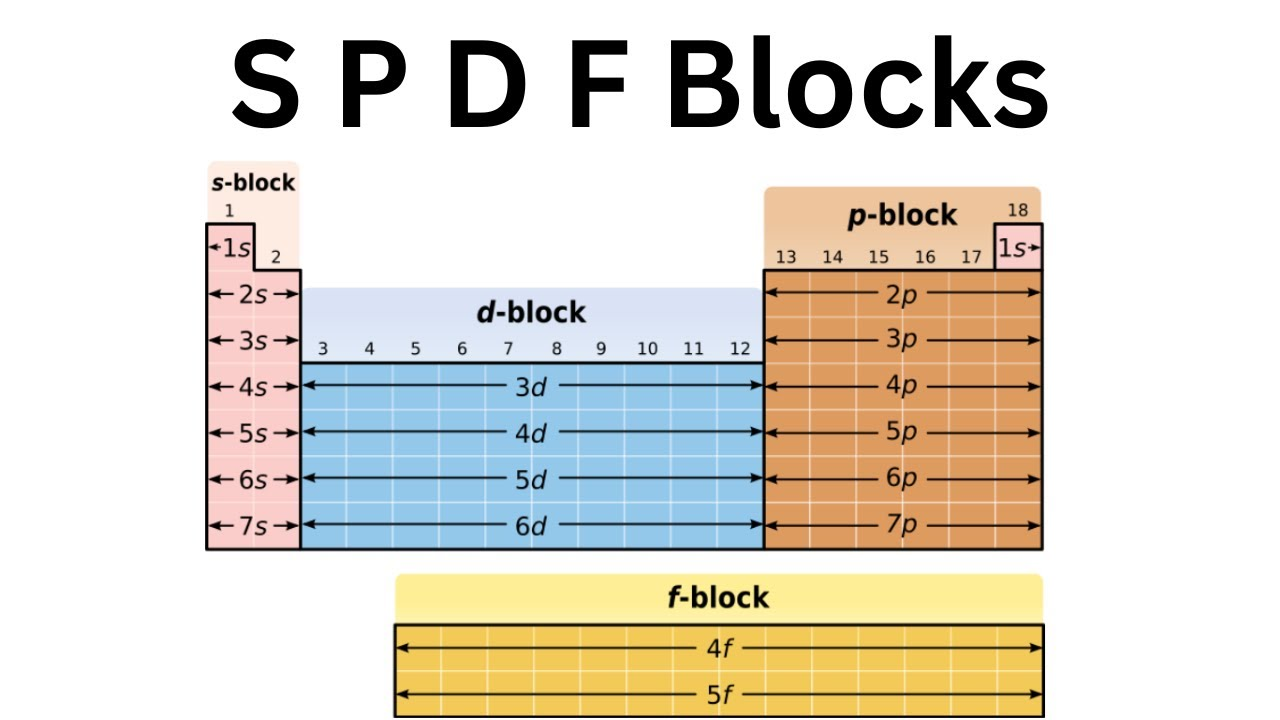
block indicates what sublevel electrons are being added to
d block elements are also known as transitional elements
electron shielding
inner electrons repel the valence electrons, shielding the valence electrons from the attractive force of the nucleus
trends in electron shielding
electron shielding is constant across a period
electron shielding increases down a group
effective nuclear charge
the effective charge experienced by valence electrons
it is the nuclear charge minus the charge of the shielding electrons
effective charge is constant across a period
effective charge increases down a group
(basically same trend as electron shielding)
trends in atomic radius
atomic radius decreases across a period
atomic radius increases down a group
trend in ionic radius across period 3
ionic radius decreases across period 3 for ions of Na, Mg, Al, Si
there is a large jump in ionic radius at P, followed by a decrease for ions of S, and Cl
positive ions have smaller ionic radius than original atom, and negative ions have larger ionic radius
first ionisation energy
energy required to remove one mole of electrons from one mole of gaseous neutral atoms
trend in 1st ionisation energy
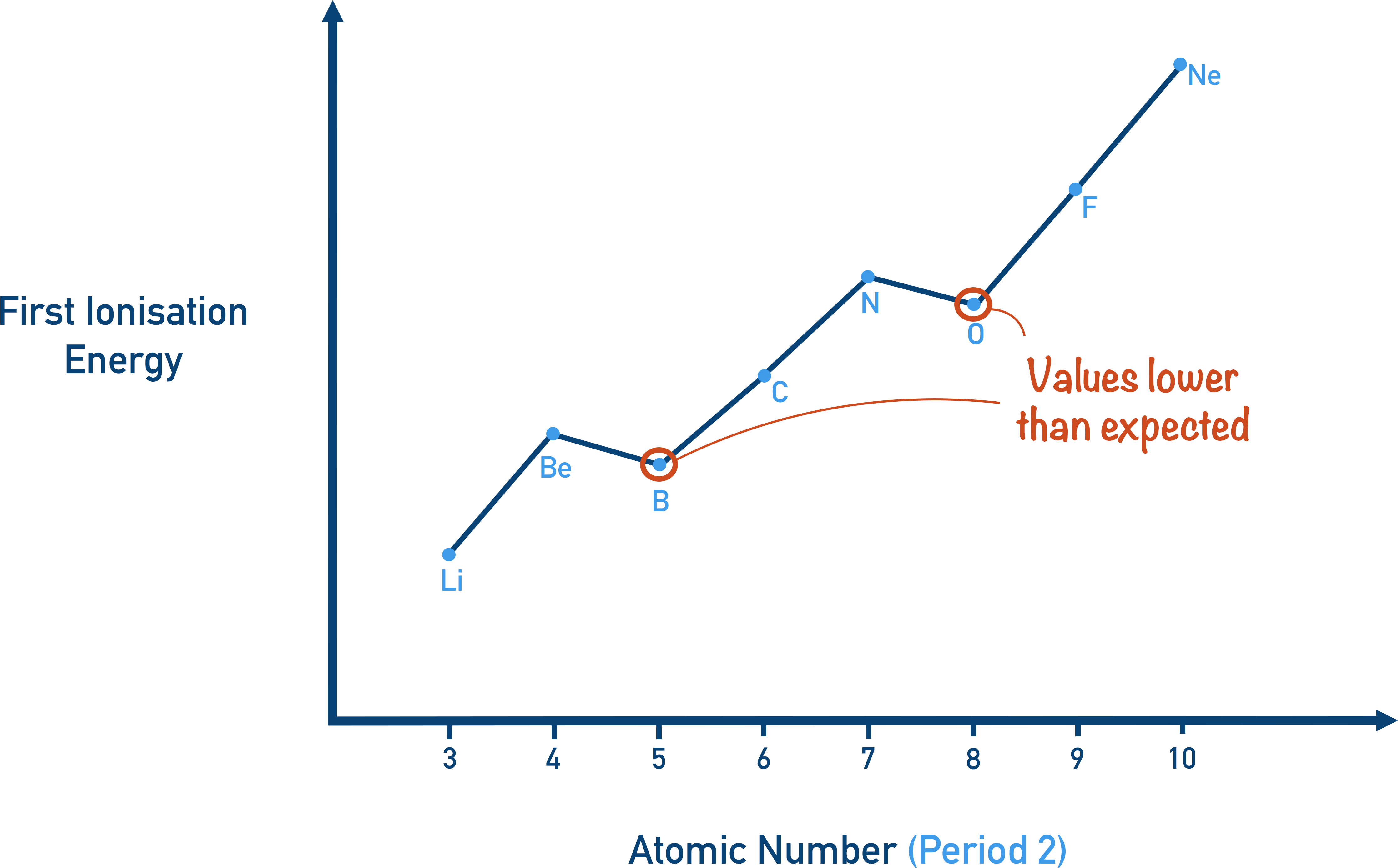
across a period the first ionisation energy increases, as the number of protons increases, which decreases ionic radius, and increases attraction
large jump down when going up a principal energy level
discrepancy when electron is removed from new subshell, as it easier to remove electron from more energetic subshell
discrepancy when electron is removed from subshell which has 1 pair, as it easier to remove electron that is repelled by another one
electron affinity
1st electron affinity is the energy released when one mole of electrons is added to one mol of neutral gaseous atoms (negative)
2nd electron affinity is positive due to extra repulsion when trying to add an electron
trends in 1st electron affinity
greater electron shielding and greater ionic radius reduces the energy released
energy released decreases down a group
energy released is more for nonmetals than for metals