Electrophilic Aromatic Substitution
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
Halogenation
rings react w/ Br or Cl in presence of Lewis Acid: FeCl3, FeBr3, AlBr3, or AlCl3
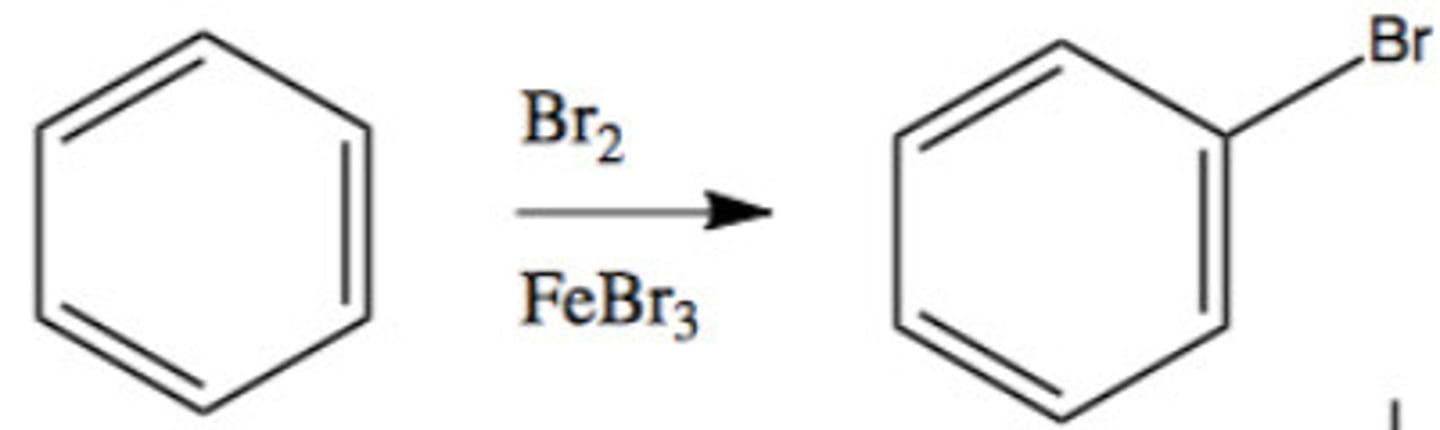
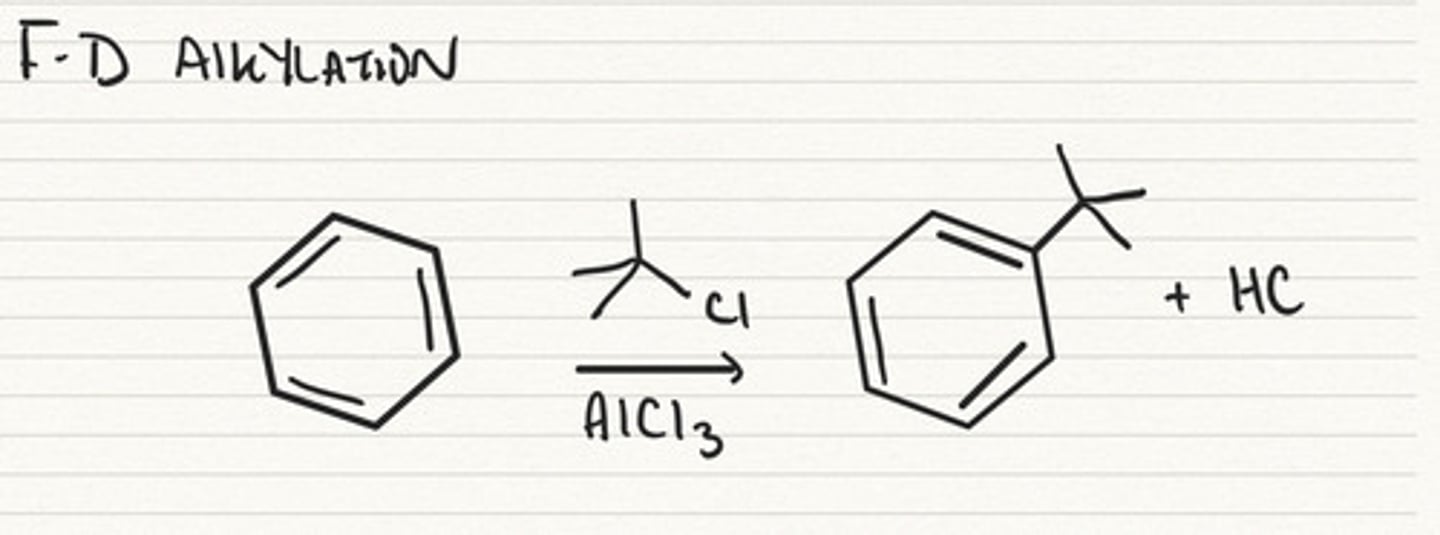
Friedel-Crafts Alkylation
R-X (Cl, Br, etc.), adds R group and removes the X. Occurs w/ AlCl3
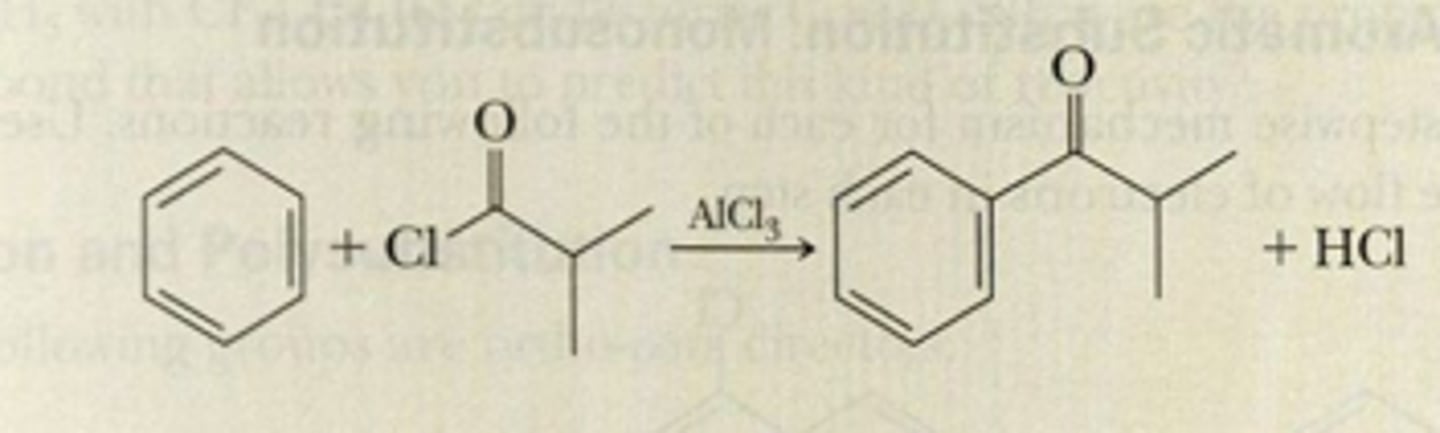
Friedel-Crafts Acylation
RCO-X (Cl, Br, etc.), adds RC=O group and removes X. Occurs w/ AlCl3
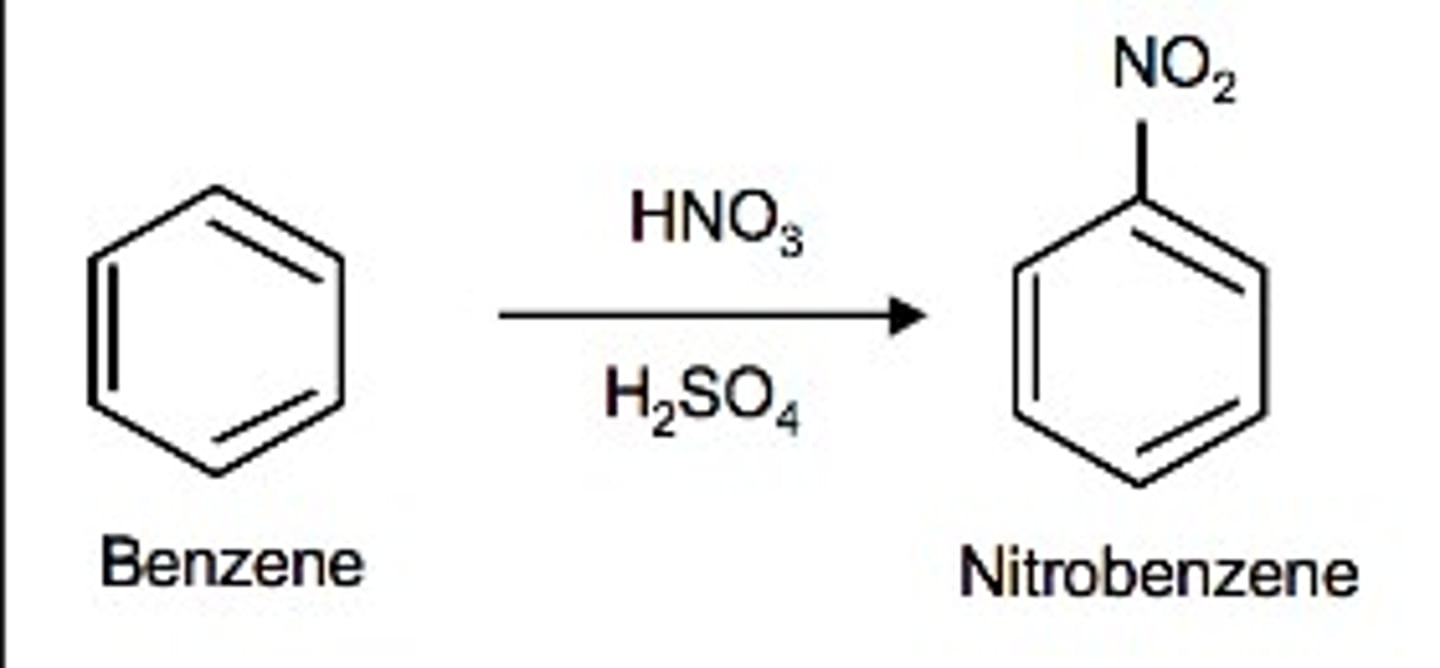
Nitration
the substitution of a nitro group (-NO2) for a hydrogen on an aromatic ring. Occurs w/ HNO3 in H2SO4
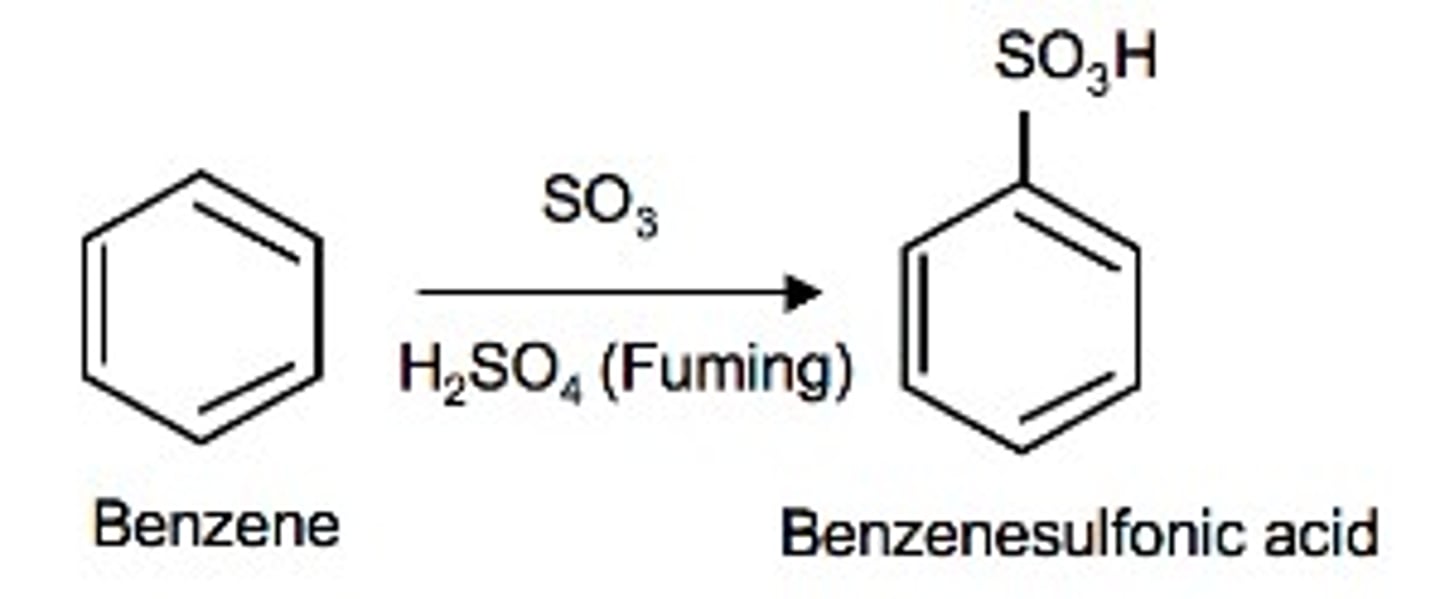
Sulfonation of Benzene
the substitution of a SO3 for a hydrogen, occurs in "fuming" H2SO4. *Reversible w/ dilute H2SO4
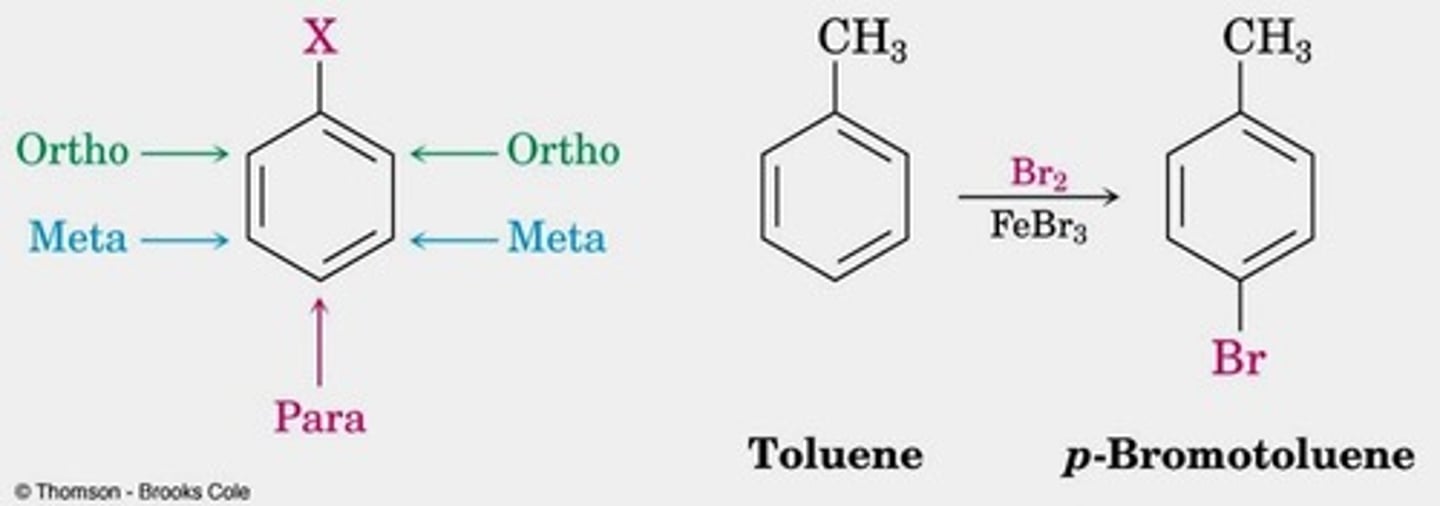
Polysubstituted Benzenes two ortho/para
the more activating substituent wins for directing (EDG).
Polysubstituted Benzenes ortho/para with meta
the ortho/para directing group wins
Two meta-directing groups
the new substituent locates at the position meta to the stronger deactivating group
Aniline w/ lewis acid present
no reaction
Aniline w/ strong acid like H2SO4
meta directing product w/ protonated amine
Aniline and phenol in the absence of an acid
highly active in EAS