SAR of beta-agonists and anti-muscarinic
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
What neurotransmitter(s) act on adrenergic receptors?
Adrenaline and noradrenaline
What neurotransmitter(s) act on muscarinic receptors?
Acetylcholine
Where are adrenergic and muscarinic receptors located?
Smooth muscle and cardiac muscle in the autonomic nervous system
What receptor do beta-2 agonists act on?
Adrenergic receptors (beta-2)
What receptor do antimuscarinics act on?
Muscarinic receptors
Describe the general role of salbutamol and ipratropium
These are short-acting (short-onset and short duration of action) bronchodilators. Therefore they provide short-term relief
They are used to treat symptoms of asthma and COPD
Describe the general process of drug development
We start with a lead compound, establish important functional groups, their arrangement in space and SAR by synthesising compounds closely related to the lead.
Then we make specific structural modifications to make it a selective agonist or antagonist.
Then we use more medicinal chemistry for final optimisation of the structure.
What are some approaches we use in medicinal chemistry to make a selective agonist or antagonist?
1. Chain extension
2. Conformational restriction
3. Group shifting
4. Chiral switching
What is a lead compound?
A prototype chemical structure/series of structures with the
desired biological activity
What was the lead compound for anti-muscarinics?
Muscarine - natural product
and
Acetylcholine - natural agonist for muscarinic receptors
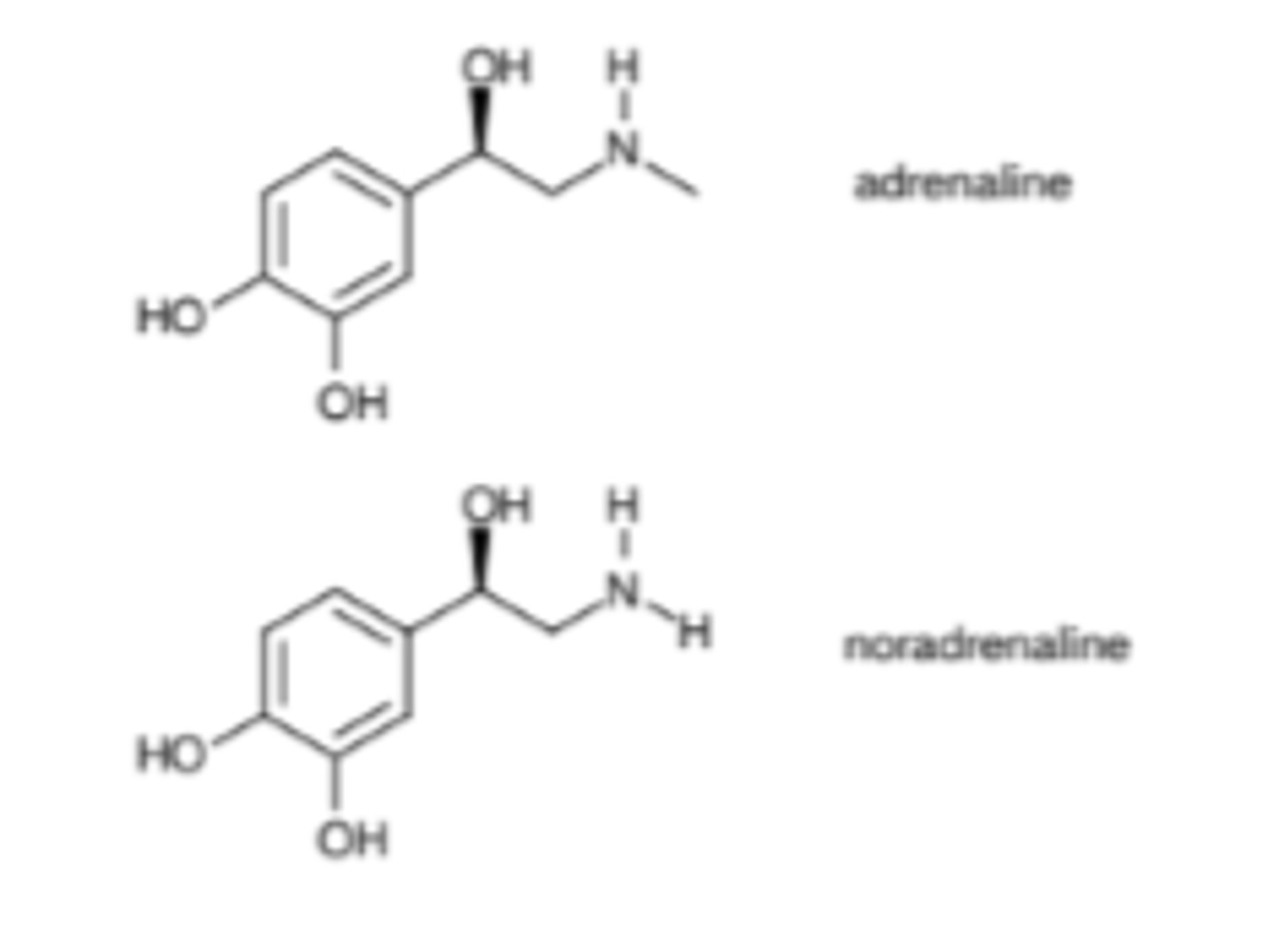
What was the lead compound for beta-2 agonists?
Adrenaline (hormone) and noradrenaline (neurotransmitter) - natural agonist for beta-2 receptors
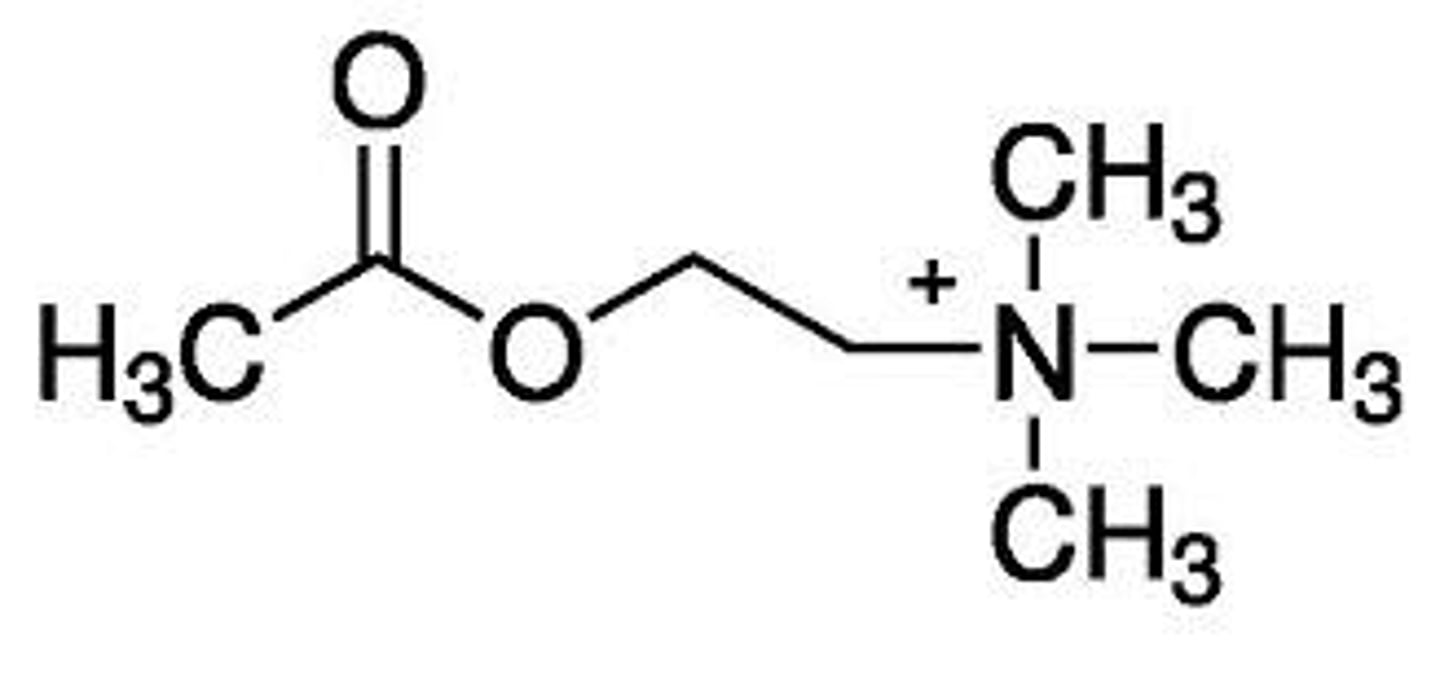
What are the key functional groups in acetylcholine?
Ester
Quaternary nitrogen
(Distance between the two groups is also important)
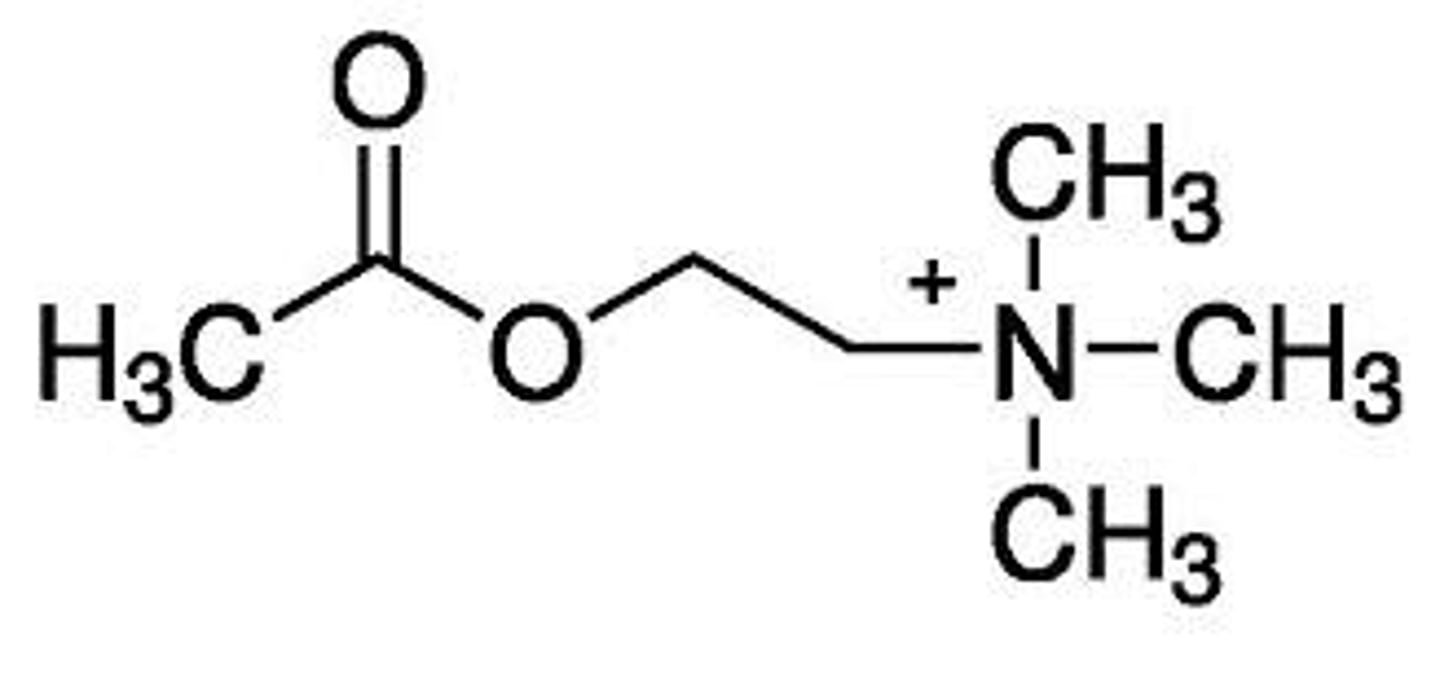
Describe the SAR for acetylcholine
- The oxygens in ester group are involved in hydrogen bonding (act as hydrogen bond acceptors).
- We see the ester group interacts with amino acid residue group of Asn-617
- The ester group cannot be large because it is a tight fit in the muscarinic receptor (size limitation).
- The quaternary Nitrogen is involved in ionic bonds.
- The distance between the quaternary nitrogen and the ester is important for binding of Ach to the muscarinic receptor
- Methyl groups (attached to N) interact with hydrophobic residues in the receptor to form van der Waals bonds.
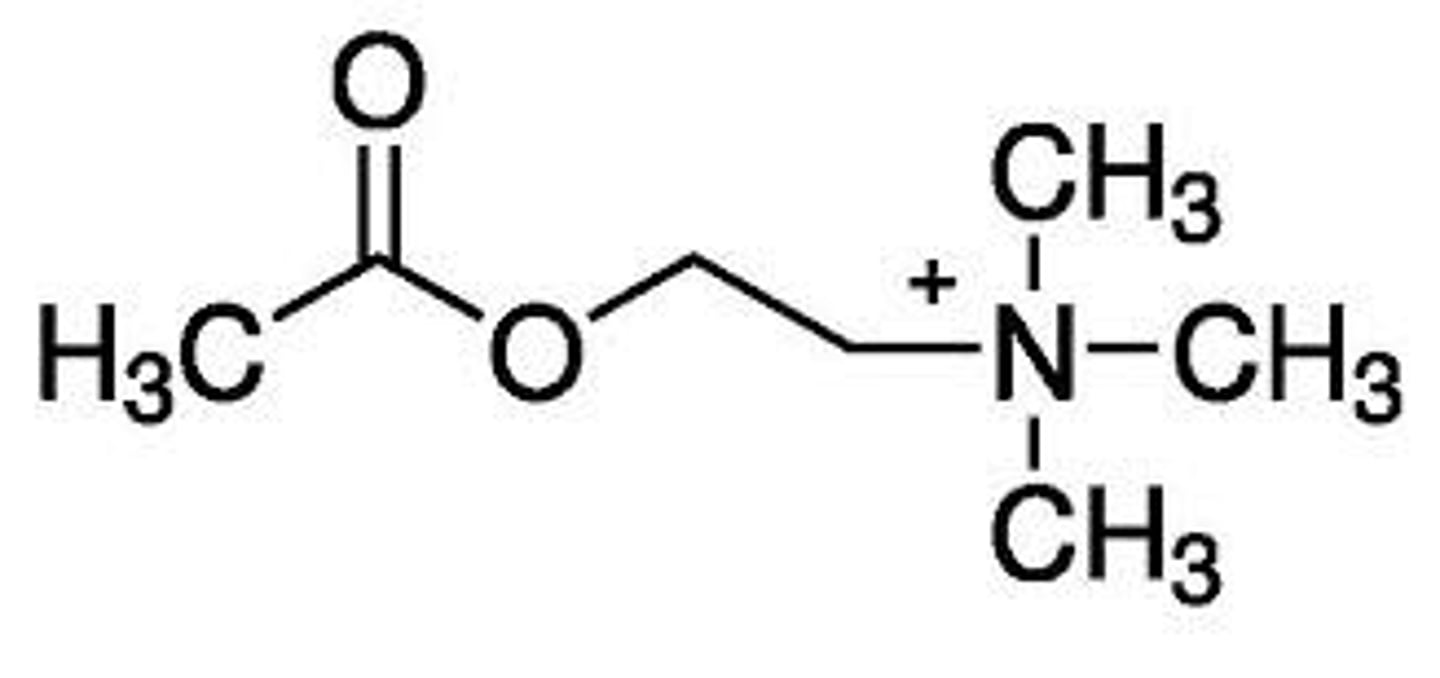
Describe the different conformations acetylcholine can adopt
Ach is a flexible molecule. There is free rotation around the sigma bonds (of which there are several). This means Ach can adopt many different conformations which will move the ester and N+ in different spatial arrangements of each other.
The active conformation does not necessarily equal the most stable conformation (Ach may adopt a different conformation to bind to different receptors).
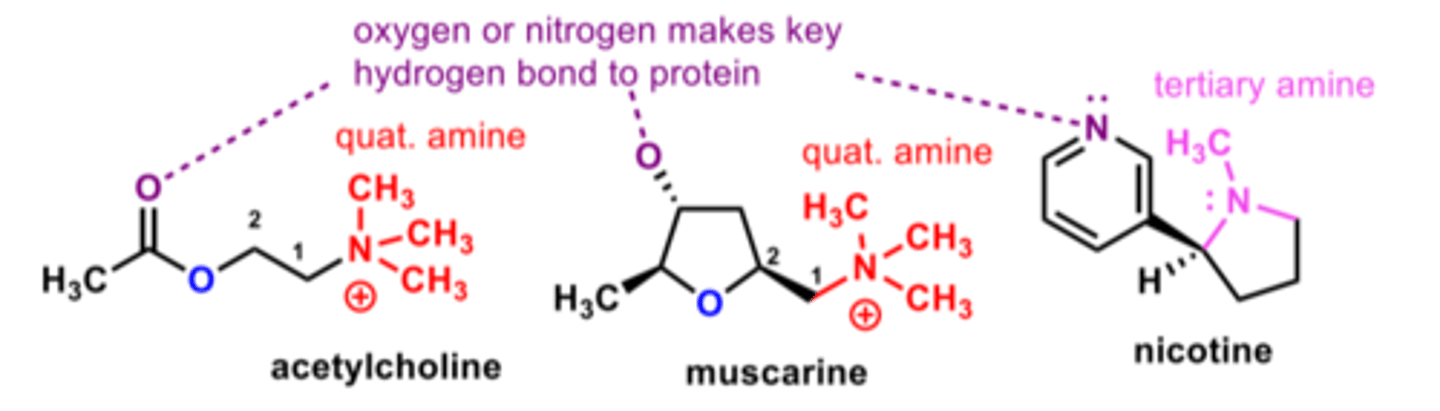
What are the two types of cholinergic receptors?
Nicotinic and muscarinic
What are the natural agonists for nicotinic and muscarinic receptors respectively?
Nicotine activates cholinergic
receptors (cholinergic) at nerve synapses
and on skeletal muscle nicotinic receptor.
Muscarine activates muscarinic receptors (cholinergic) on smooth muscle and cardiac muscle.
Compare muscarine and acetylcholine
- Quaternary N (N+) is present in both structures
- An ester is present in both compounds. In muscarine, the ester group is locked in a cyclic 5 membered ring, whereas in acetylcholine there is an aliphatic ester.
- Muscarine doesn't have free bond rotation (bonds locked within a ring), whereas acetylcholine does.
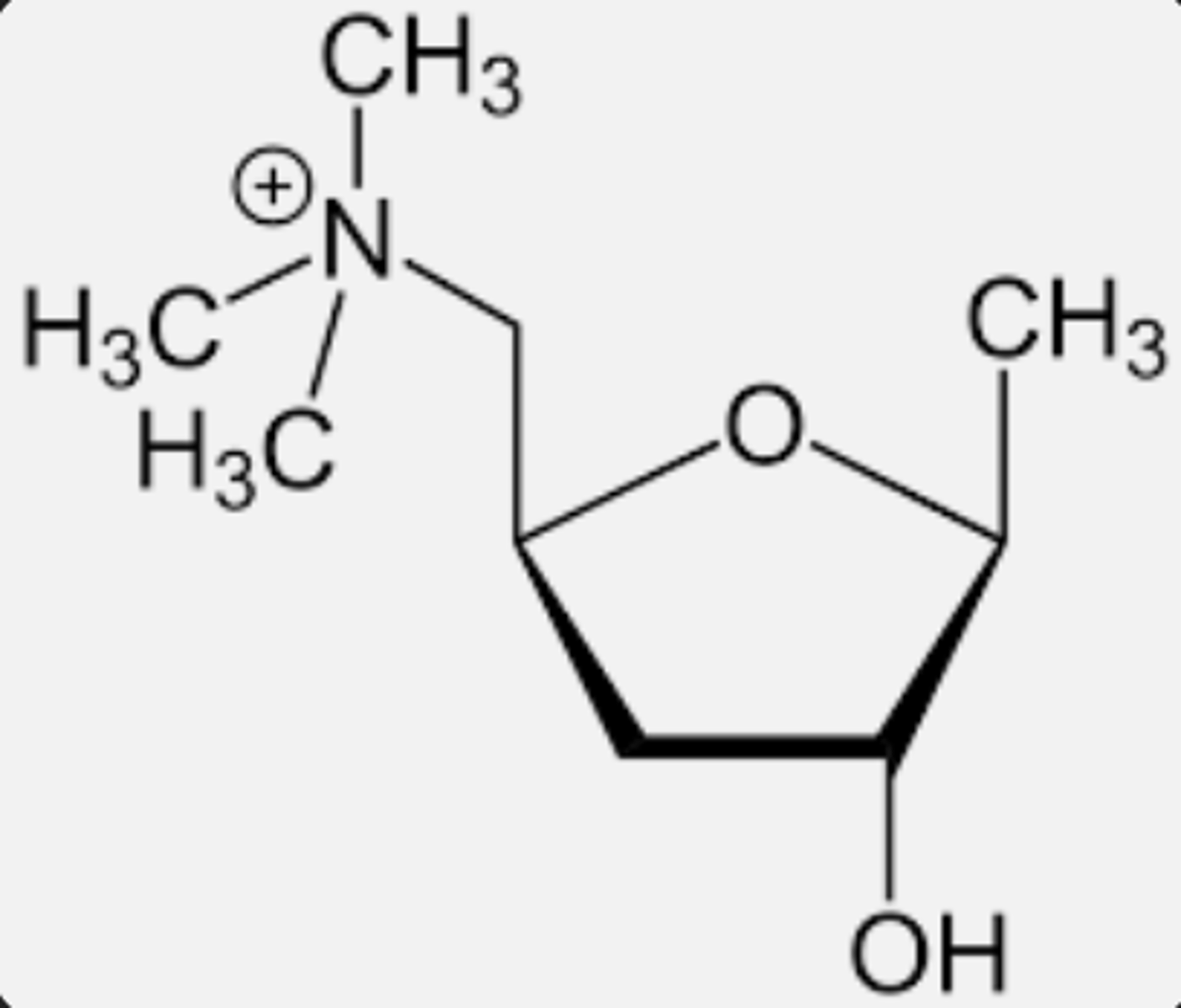
What is the significance of having sigma bonds in muscarine locked in a ring?
By having the sigma bonds locked within the ring, it defines the distance separating the ester and quaternary nitrogen in the pharmacophore. This restricts the number of conformations which muscarine can form.
Therefore the functional groups must be held at a specific distance.
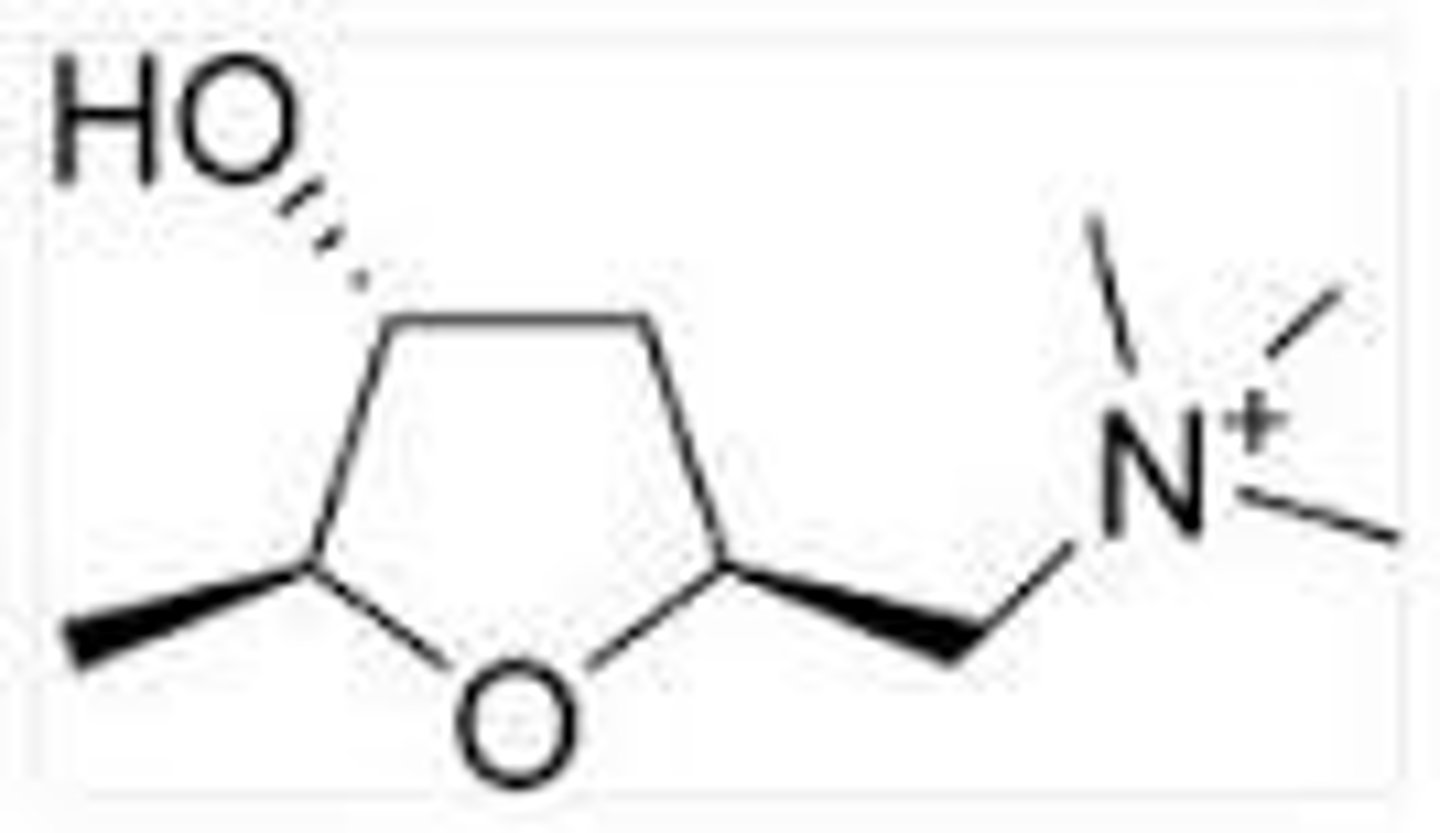
What were the natural product leads in the development of a muscarinic receptor antagonist?
Atropine
Hyoscine (scopolamine)
Compare the structure of atropine to acetylcholine
Atropine has the 2 important functional groups found in acetylcholine. It has an ester and a tertiary amine which could become a quaternary N (N+) at physiological pH.
The relative positions of the ester and the nitrogen are similar to one particular conformation of Ach.
The difference between them is that atropine has an additional hydrophobic group (phenyl/ side chain benzene) which allows it to bind tighter and stronger to the muscarinic receptor by forming van der Waal forces. This allows it to act as an antagonist
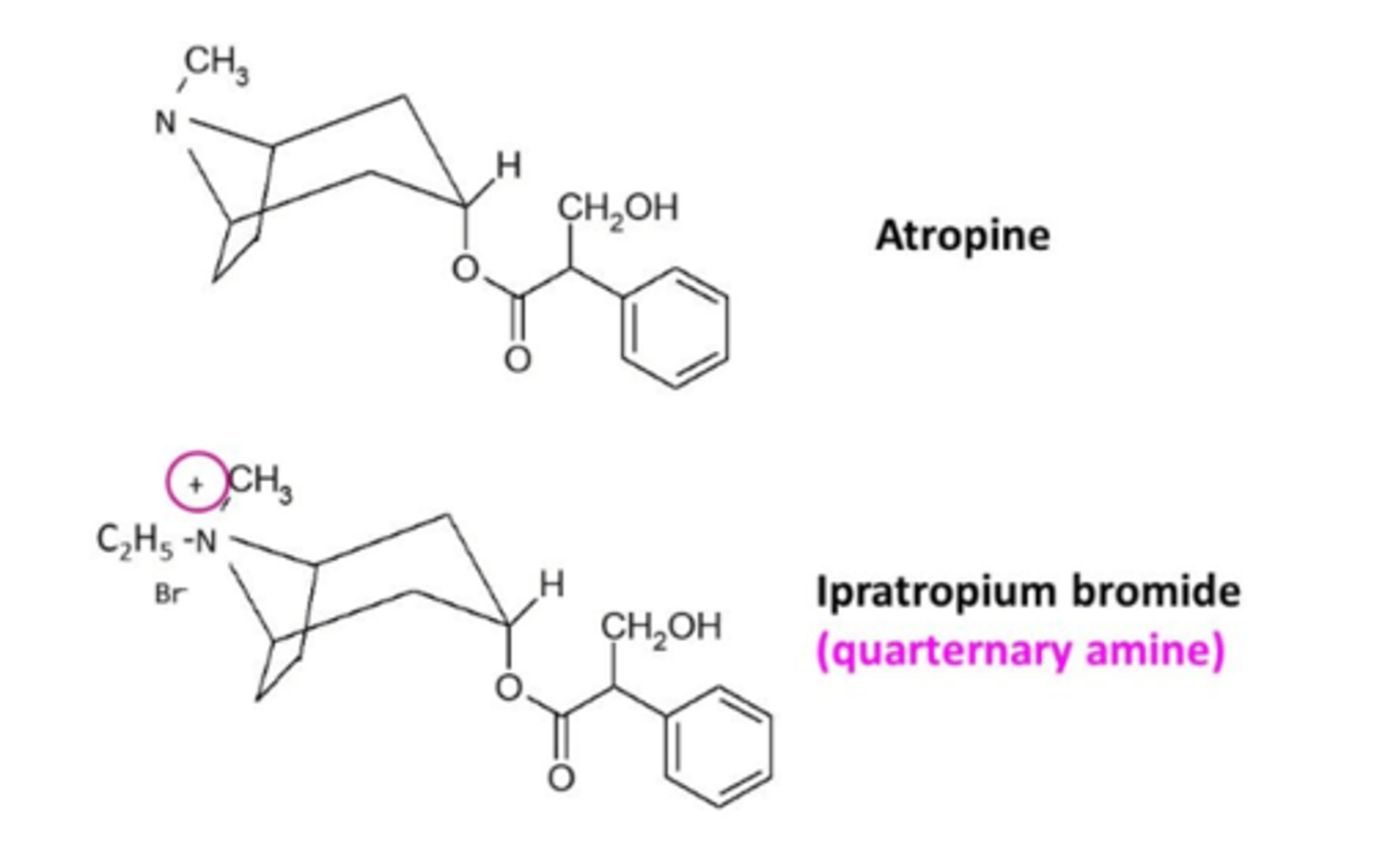
What is an important difference between atropine and ipratropium?
Ipratropium has a quaternary nitrogen whereas atropine has a tertiary amine.
This means it has no CNS side effects because it cannot cross the blood brain barrier.
It cannot cross the blood brain barrier because it has the quaternary N (N+)
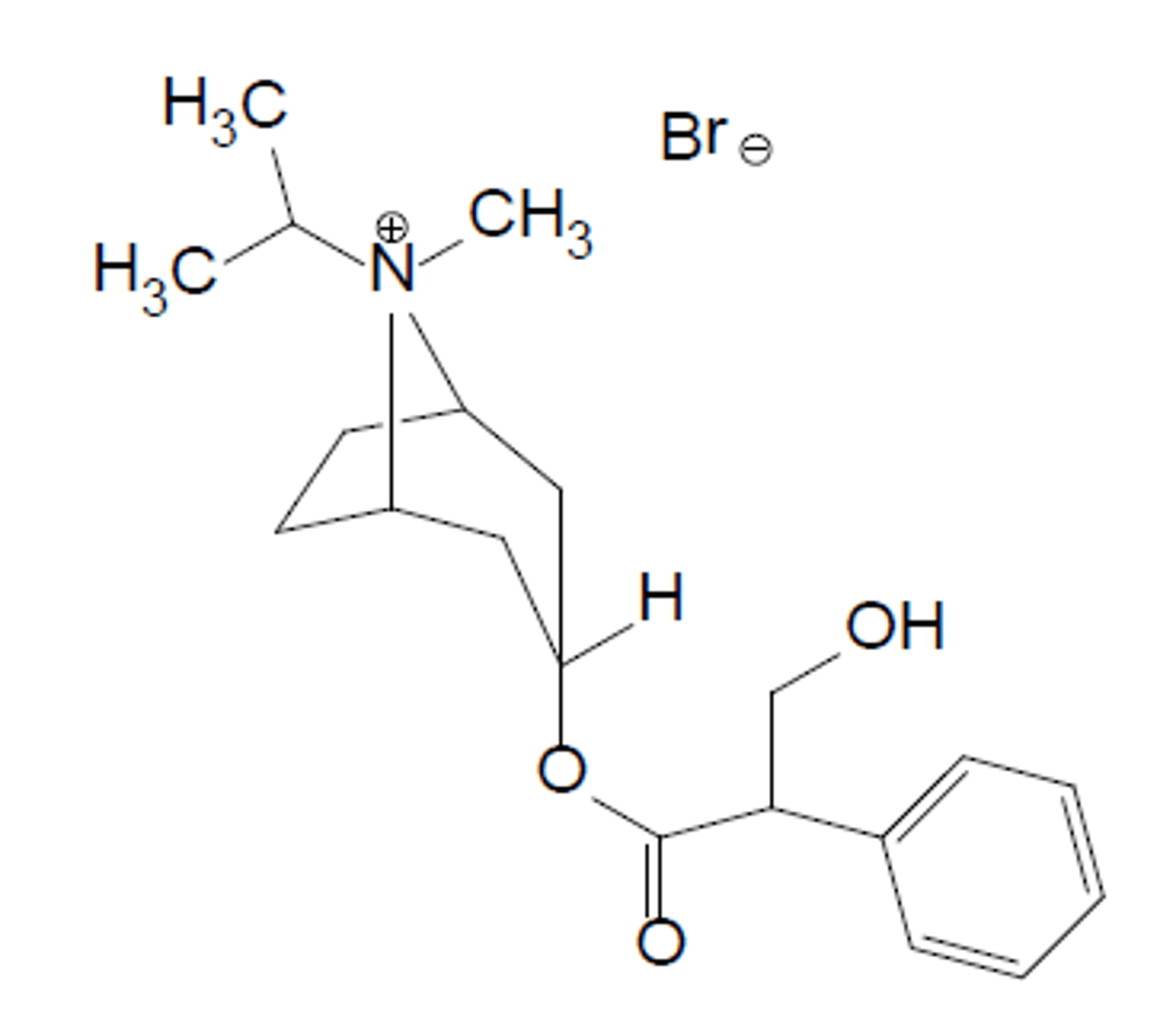
Describe the SAR for muscarinic receptor antagonists
It must have an ionised tertiary amine or a quaternary nitrogen (N+).
An ester
An additional hydrophobic group next to the ester unit (aromatic or heteroaromatic group) to form van der Waals interactions with residues outside of Ach binding point to form an a antagonist
What is the effect of activating a beta-2 adrenergic receptor?
Activation of beta-2 adrenergic receptors relaxes smooth muscles.
Where do beta-2 adrenergic receptors predominate?
Beta-adrenoceptors predominate in the airways.
Describe the general structure of catecholamines
1. Catechol (1,2 diphenyl)
2. Alkylamine
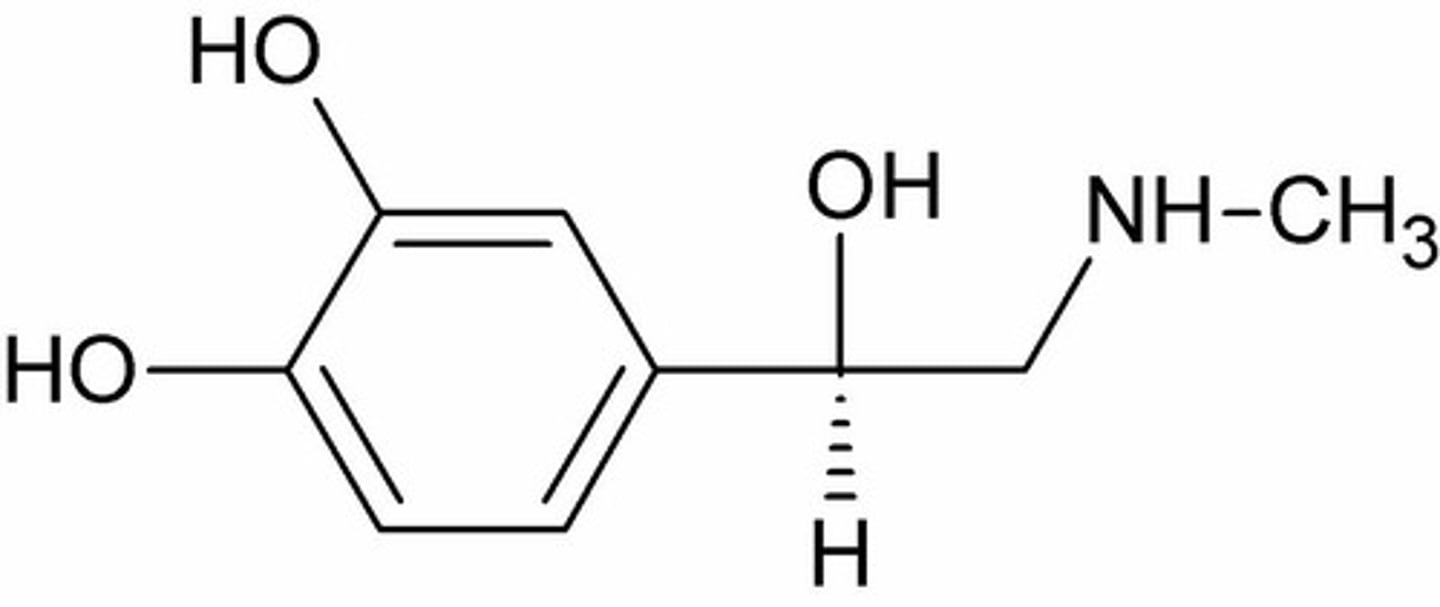
What are the key functional groups of catecholamines as adrenergic agonists?
- Phenolic hydroxyl group
- Aliphatic hydroxyl
- Protonated amine
- N-alkyl group
- Aromatic ring
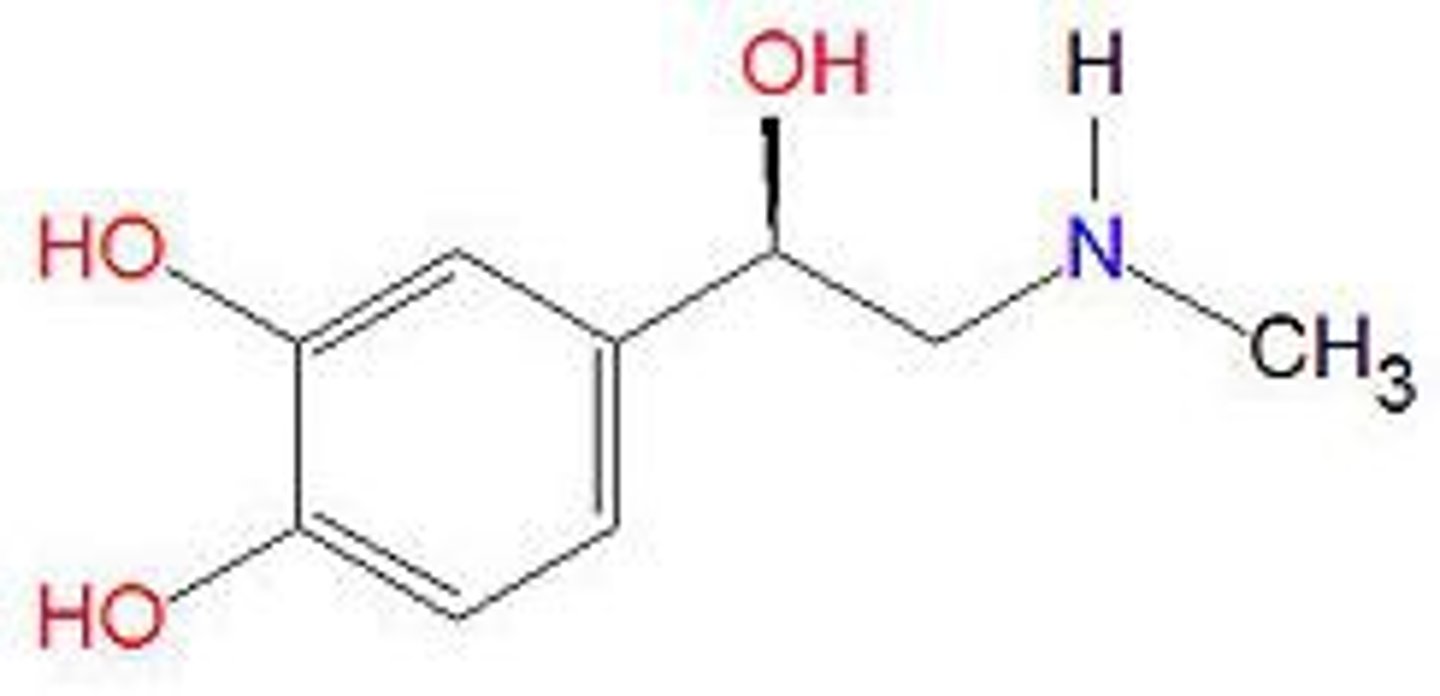
Describe SAR for catecholamines
- Phenolic hydroxyl groups: 2 hydroxyl groups for hydrogen bonding (with serine residues, serine residues are close together)
- Aliphatic hydroxyl: 1 hydroxyl group (aliphatic) for hydrogen bonding
- Chiral centre: both enantiomers are active for bronchodilator activity but R-enantiomer is more active
- Protonated amine (NHR2+) for ionic bonds (makes ionic bonds with -COO- in aspartic acid).
- Larger N-alkyl groups lead to selectivity for b-adrenoceptors.
- Aromatic ring is hydrophobic so forms van der Waals interactions with the binding site.
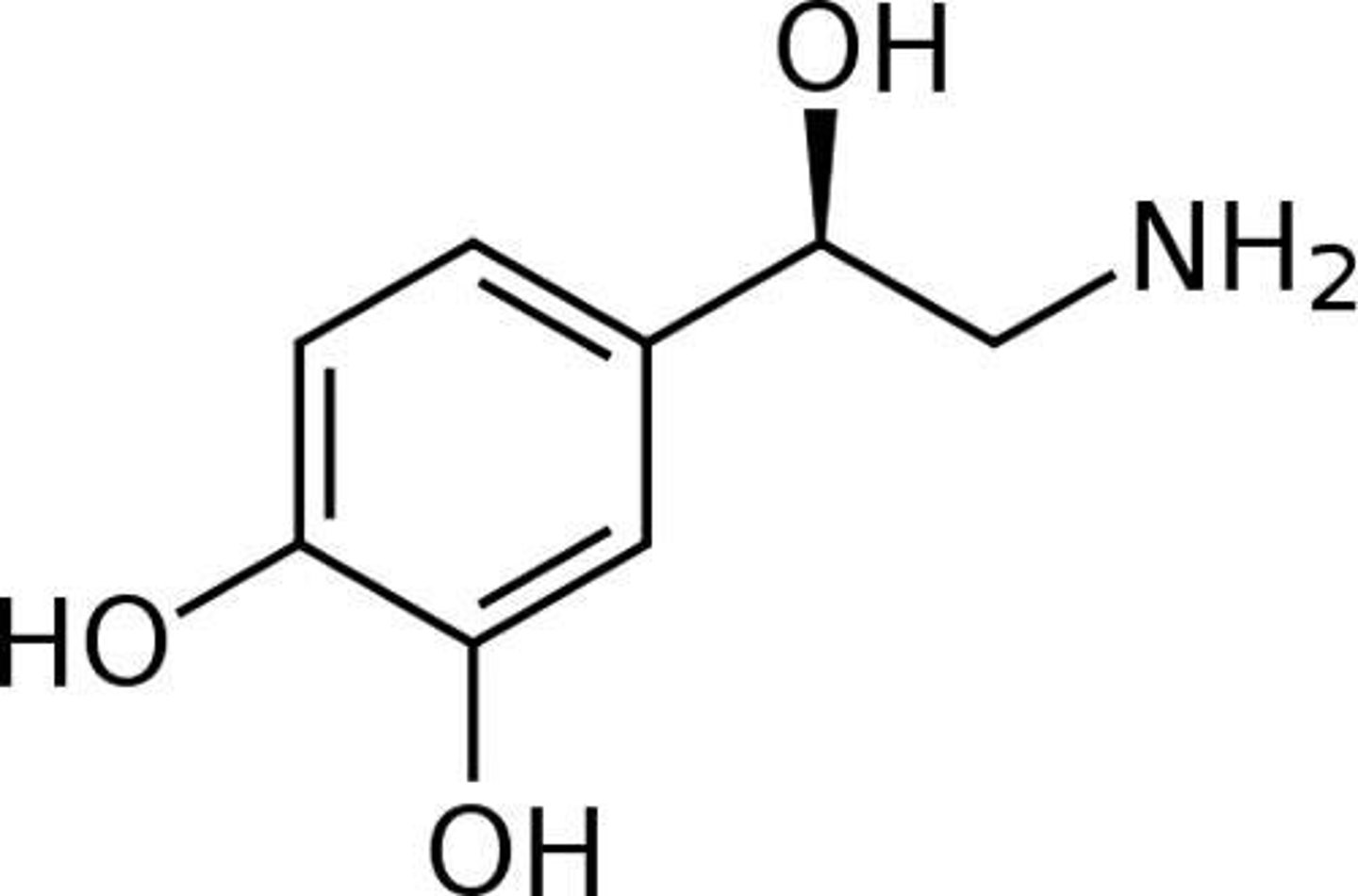
Compare the structure of isoprenaline to catecholamines (noradrenaline, adrenaline)
The key modification compared to natural ligand (catecholamines - noradrenaline and adrenaline) is that there is chain extension from the amine. This leads to greater selectivity for beta-adrenoreceptors.
BUT it has cardiovascular side effects because it has no selectivity between beta subtypes so can also act as an agonist for beta-1 adrenergic receptors.
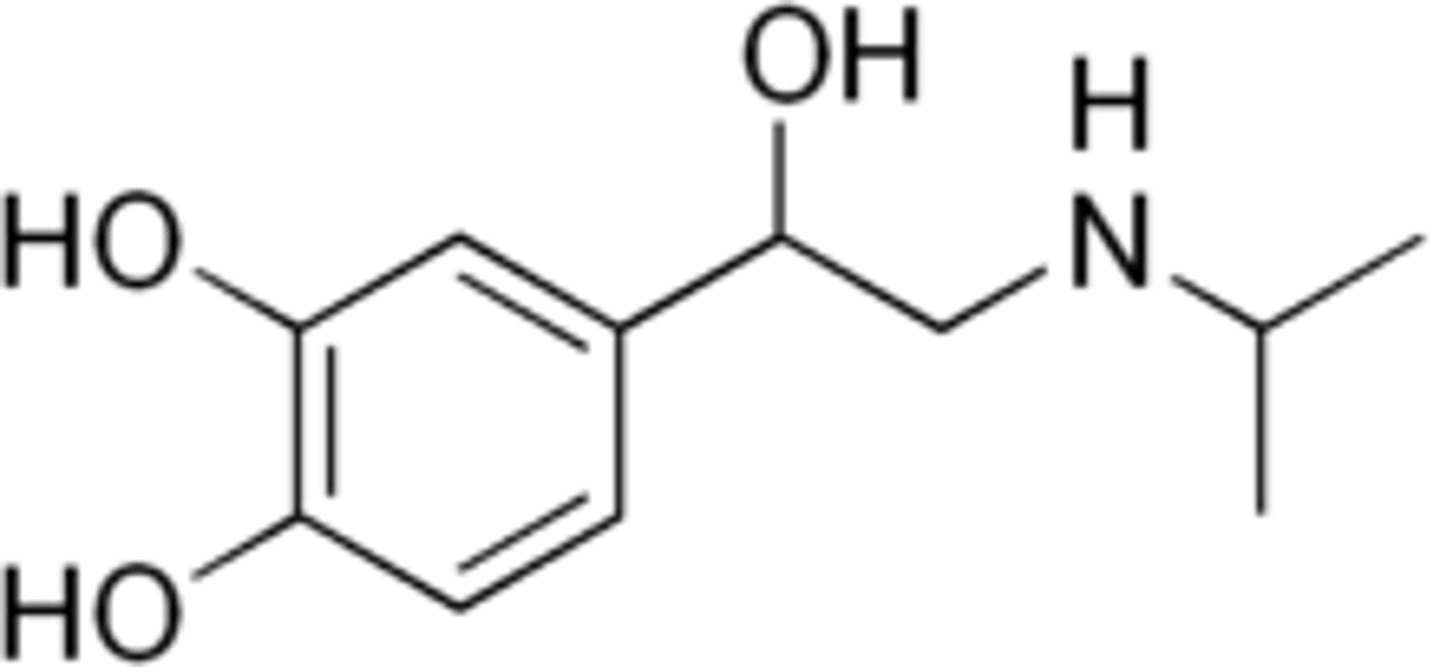
Compare the structure of isoetharine to catecholamines (noradrenaline, adrenaline)
The main difference is that isoetharine has an additional of alkyl group between the catechol and amine functional group.
But, this is bad because it makes it a good substrate for COMT, so O-methyl transferases add methyl group to the phenolic hydroxyl group.
This is detrimental for receptor binding as phenolic OH can no longer act as a H bond donor/acceptor for serine residues.
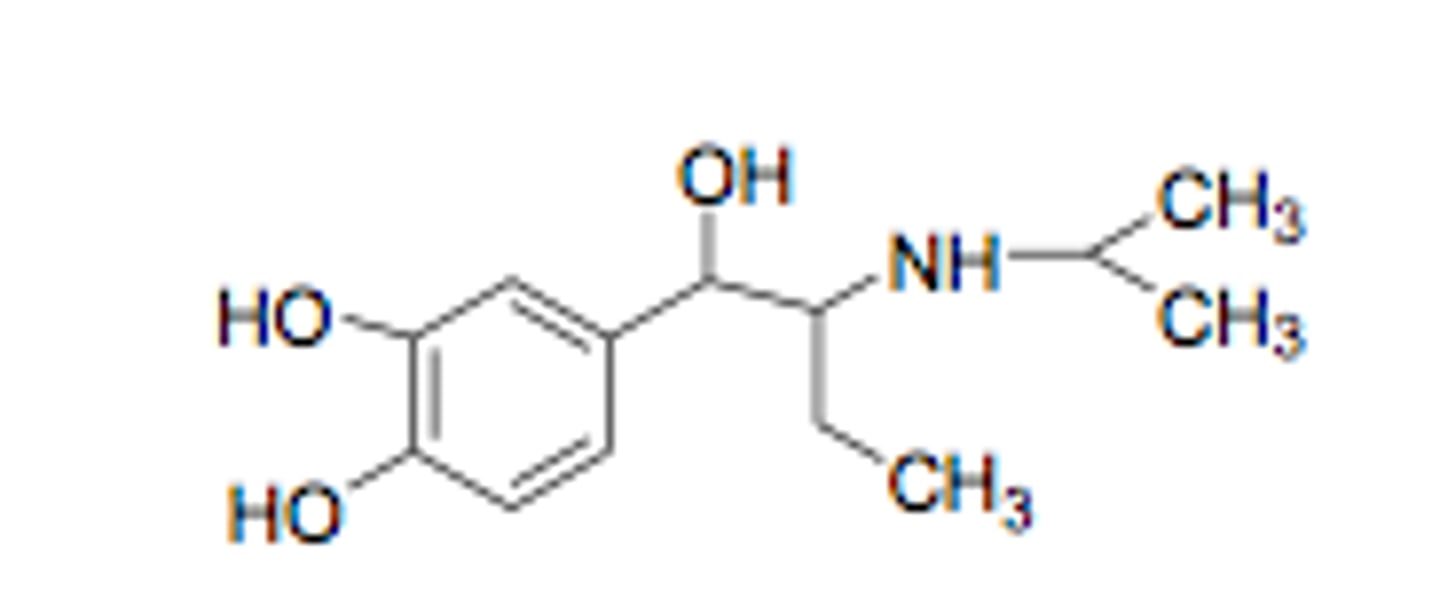
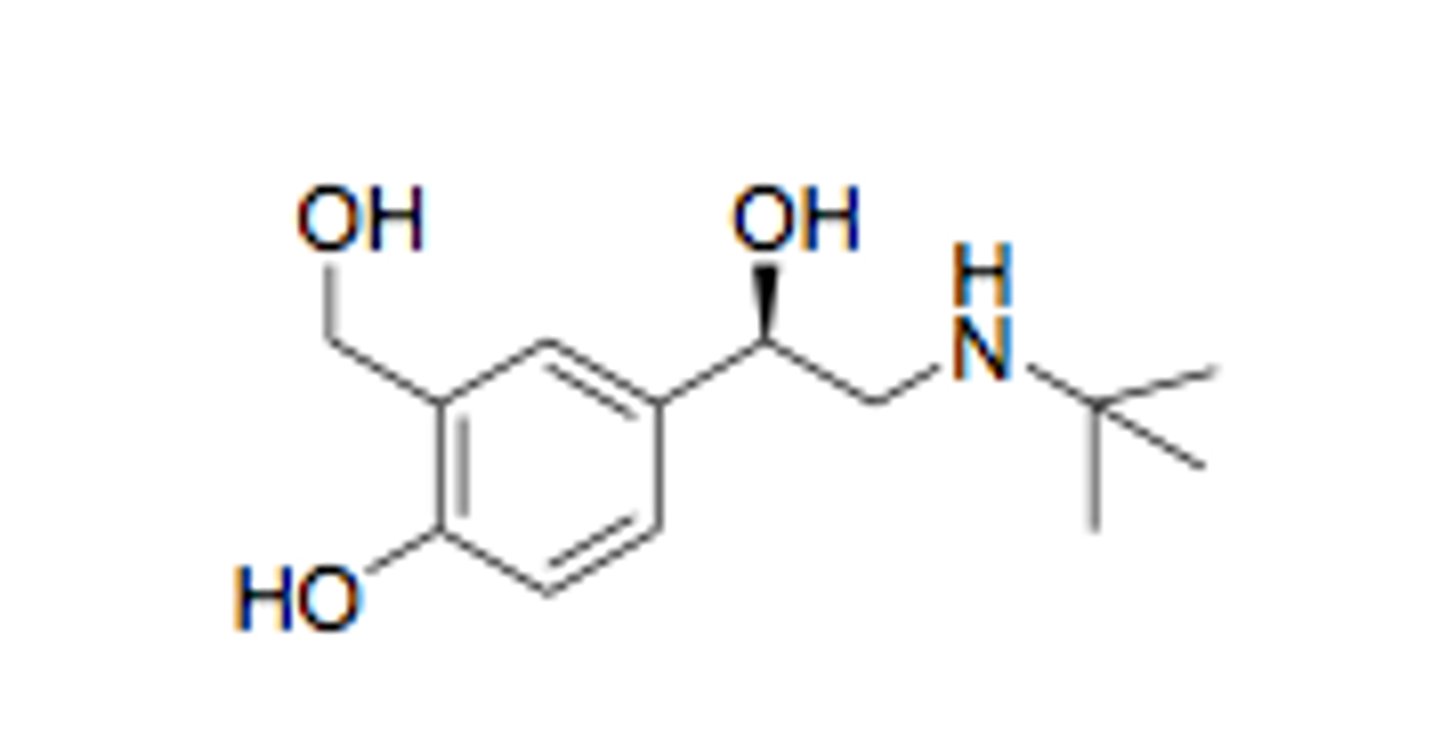
Describe the structure of salbutamol
A T-butyl group is added to the amine for beta selectivity.
One of the hydroxyl groups on the benzene ring is shifted by one carbon (group shift) which prevents the metabolism of the drug and still keeps beta-2-antagonist activity.
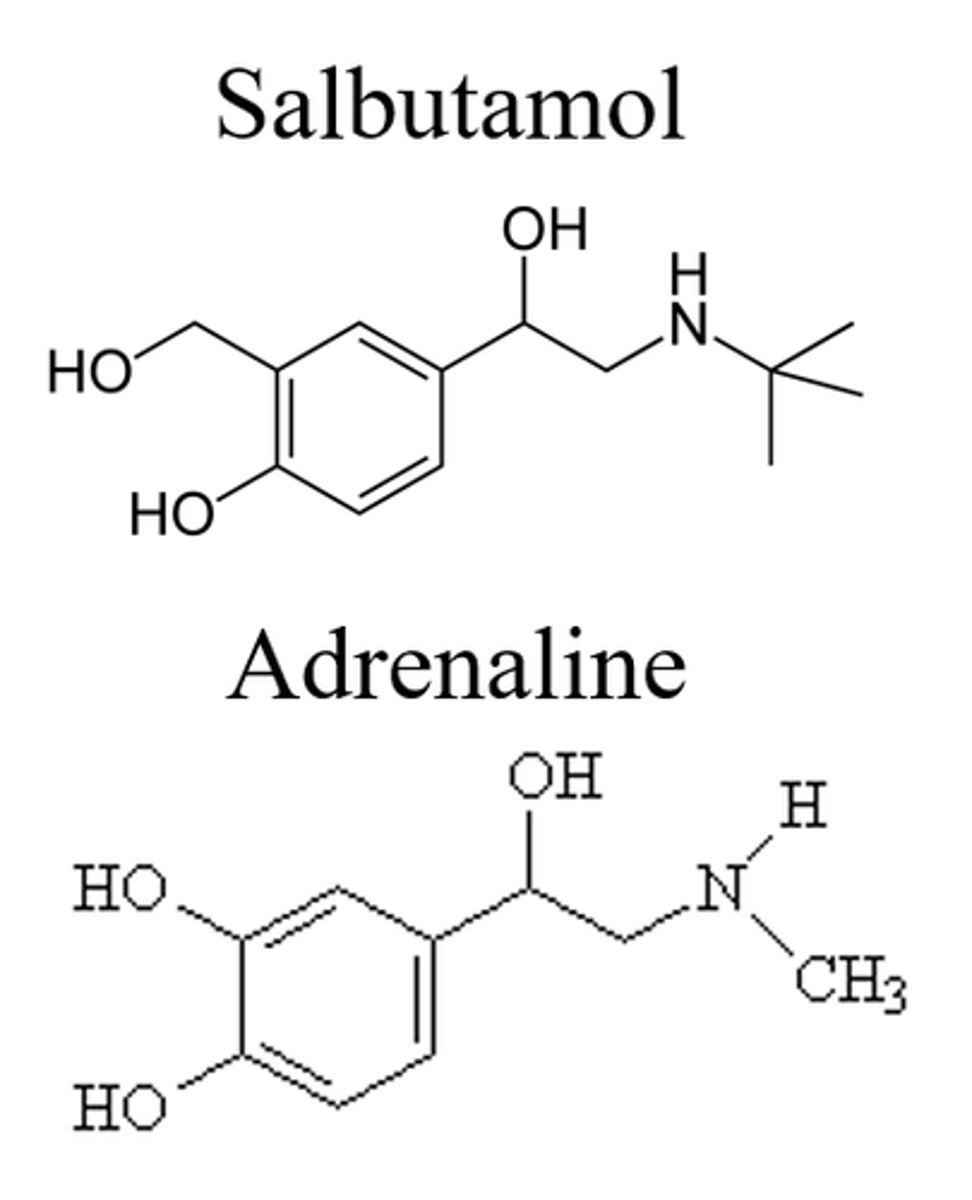
Describe the chirality of salbutamol
The carbon with the aliphatic hydroxyl group is the chiral centre.
The R enantiomer of salbutamol is the most effective form (developed separately).
Salbutamol is given as a racemic mixture
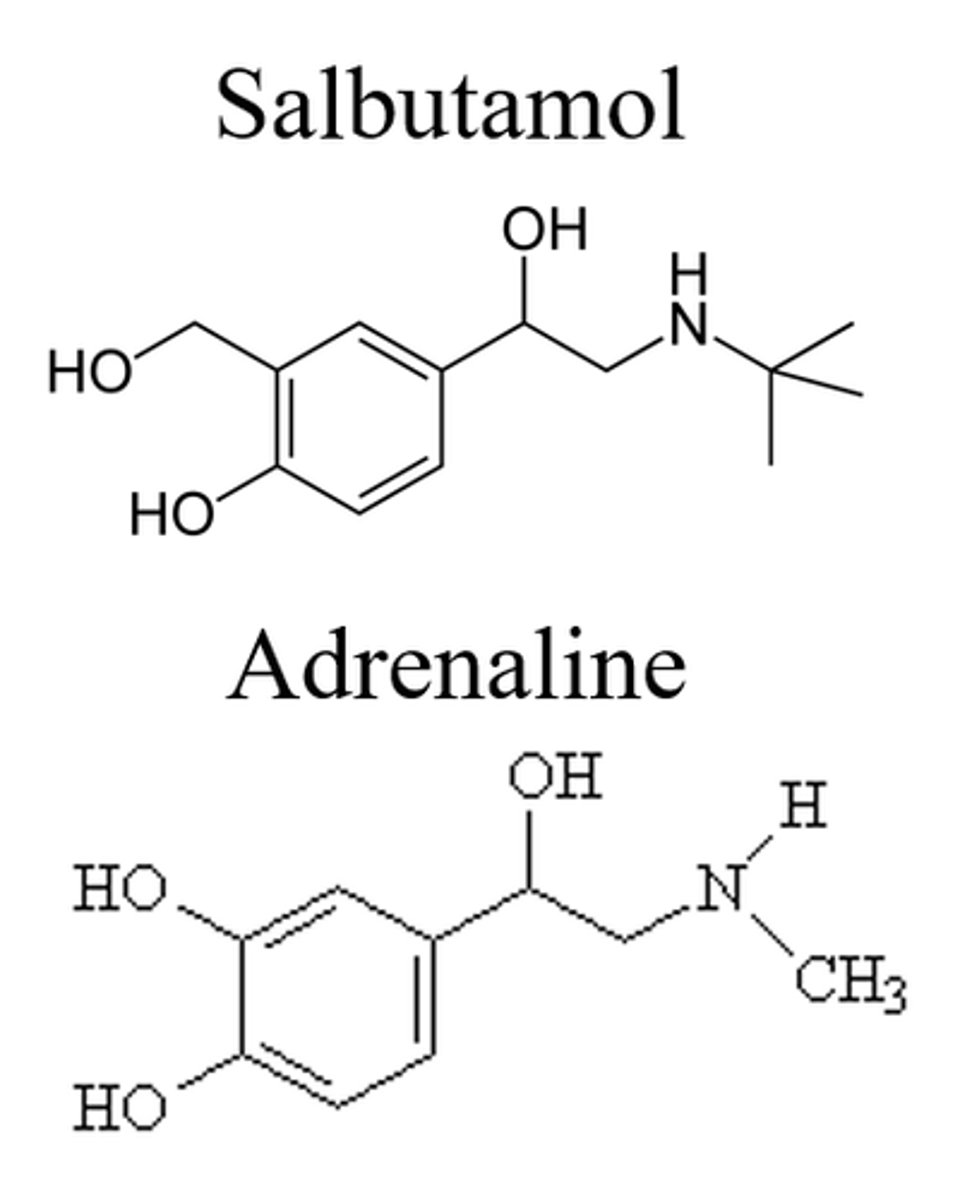
What is the name of salbutamol with only the R-enantiomer?
Levalbuterol
This is another example of chiral switching (salbutamol then levalbuterol development)