Acid Base Disorders Pathophysiology (Zhang)
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
Normally, blood pH is maintained at 7.40 ([H+] of _______) with a range of 7.35 to 7.45
4 × 10-8 M
_____ is an arterial blood pH < 7.35
acidemia
_____ is an arterial blood pH > 7.45
alkalemia
pH of 6.7 ([H+] of ____ M), representing a ___% increase in hydrogen ion concentration (Hint: pH = -log[H+])
2 × 10-7, 500%
pH of 7.7 ([H+] of ____ M), representing a ___% increase in hydrogen ion concentration (Hint: pH = -log[H+])
2 × 10-8, 50%
What are the three methods of Acid-Base Homeostasis?
Extracellular buffering, Ventilatory regulation, Renal regulation
What is extracellular buffering?
Rapid acting and first defense against sudden increase in H+
What is Ventilatory regulation?
hyperventilation increases CO2 elimination → decrease in PCO2 (LUNGS)
What is Renal regulation?
kidney excrete excess H+ (normal pH over a couple of days)
Where does renal regulation of bicarbonate reabsorption occur in the kidneys?
proximal convoluted tubule
Where does renal regulation of acid excretion occur in the kidneys?
Distal tubule/collecting duct
What is the pH and primary disturbance of metabolic acidosis?
decrease pH, decrease bicarbonate
What is the pH and primary disturbance of metabolic alkalosis?
increase pH, increase bicarbonate
What is the pH and primary disturbance of respiratory acidosis?
decrease pH, increase pCO2
What is the pH and primary disturbance of respiratory alkalosis?
increase pH, decrease pCO2
What is the pathophysiology of metabolic acidosis?
decrease in pH as a result of a primary decrease in serum bicarbonate conecentration
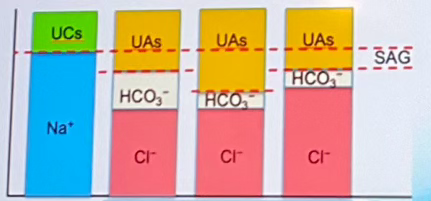
What is the serum anion gap (SAG)?
used to determine if organic or mineral acidosis occurred:


Which SAG equation can infer elevated anion gap metabolic acidosis?
SAG = [UAs] - [UCs]
What is elevated anion gap metabolic acidosis?
Increases in the anion gap (SAG) is indicative of the accumulation of unmeasured anions in ECF

Which SAG equation can infer Hyperchloremic metabolic acidosis?
SAG = [Na+] - [Cl-] - [HCO3-]
What is Hyperchloremic metabolic acidosis
SAG remains normal because bicarbonate losses from the ECF are replaced by chloride
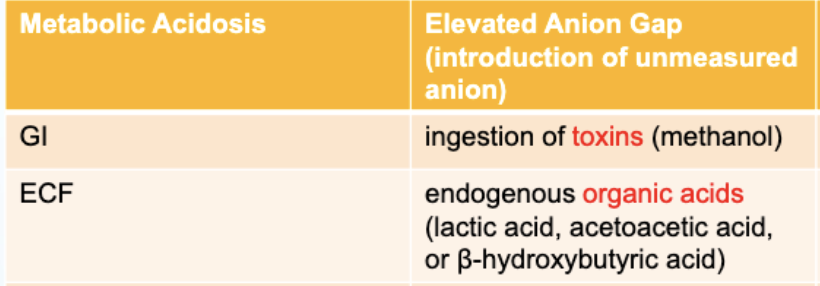
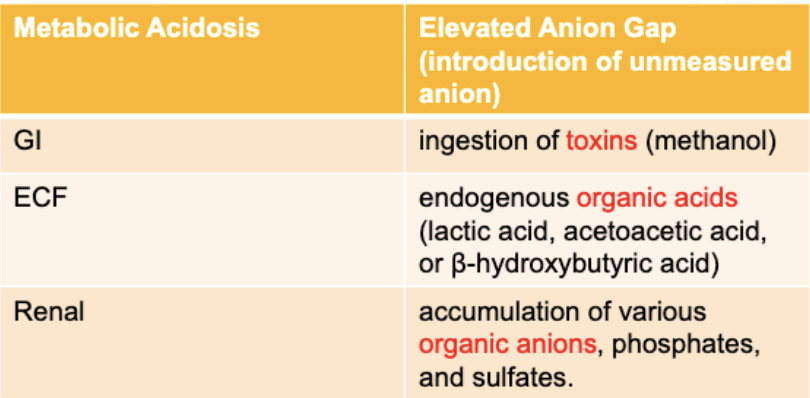
For Metabolic Acidosis-Elevated Anion Gap: What is the GI pathophysiology?
ingestion of toxins (methanol)
For Metabolic Acidosis-Elevated Anion Gap: What is the ECF pathophysiology?
endogenous organic acids (lactic acid, acetoacetic acid, B-hydroxybutyric acid)
For Metabolic Acidosis-Elevated Anion Gap: What is the Renal pathophysiology?
accumulation of various organic anions, phosphates and sulfates
For Metabolic Acidosis-Hyperchloremic: What is the GI pathophysiology?
GI tract losses / immense loss of bicarbonate (e.g. diarrhea)
For Metabolic Acidosis-Hyperchloremic: What is the ECF pathophysiology?
rapid administration of chloride-containing IV fluids
For Metabolic Acidosis-Hyperchloremic: What is the Renal pathophysiology?
renal bicarbonate wasting, impaired renal acid (hydrogen ion) excretion
What are the unmeasured organic acids in ECF that indicate an elevated anion gap?
lactic acid, acetoacetic acid, B-hydroxybutyric acid
In addition to the unmeasured organic anions that indicate an elevated anion gap caused by renal pathology, what are the other organics that indicate a gap?
phosphates, sulfates
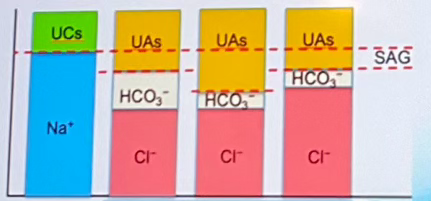
Which of the columns indicates normal pH? (labeled A-D left to right)
Column B
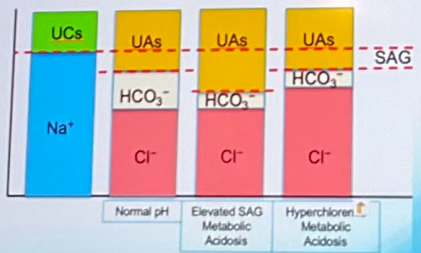
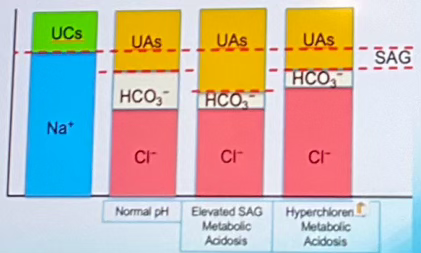
Which of the columns indicates elevated SAG Metabolic Acidosis? (labeled A-D left to right)
Column C
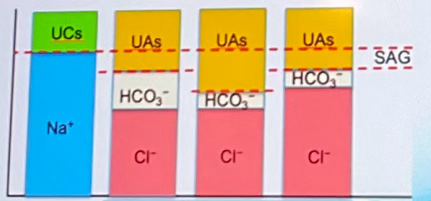
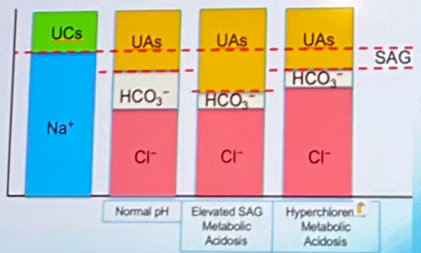
Which of the columns indicates Hyperchloremic Metabolic Acidosis? (labeled A-D left to right)
Column D
Which type of Metabolic Acidosis can also be called Renal Tubular Acidosis?
Hyperchloremic Metabolic Acidosis caused by renal bicarbonate wasting and impaired renal acid excretion
Renal Tubular disorders can involve which 2 part of the nephron?
proximal tubule and distal tubule
What are dysfunction can happen at the proximal tubule in renal tubular disorders?
failure to reabsorb filtered bicarbonate
What are dysfunction can happen at the distal tubule in renal tubular disorders?
failure to excrete H+
How is renal tubular acidosis type I ID'd
distal tubule can’t remove H+, HYPOkalemia
How is renal tubular acidosis type 2 ID'd
failure to reabsorb bicarbonate at proximal tubule
How is renal tubular acidosis type 3 ID'd
distal tubule can’t remove H+, failure to reabsorb bicarbonate at proximal tubule
How is renal tubular acidosis type 4 ID'd
distal tubule can’t remove H+, HYPERkalemia
What is the most important extracellular buffer system?
monobasic/dibasic phosphate
carbonic acid/bicarbonate
ammonium/ammonia
carbonic acid/bicarbonate
Which one responds to pH changes slowest?
renal regulation
extracellular buffering
ventilatory regulation
renal regulation
Metabolic alkalosis is caused by _____?
decreased plasma bicarbonate concentration
increased plasma bicarbonate concentration
decrease PCO2
increase PCO2
increased plasma bicarbonate concentration