lecture 4: chirality
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
isomers
set a molecules that have the same molecular formula, nut have a different arrangement of the atoms in space
constitutional isomers
atoms are arranged in a different order
different physical and chemical properties
distinctly different molecules
stereoisomers
atoms are arranged in the same order but in different 2-D orientation
line drawings in 3-D space
thick triangles: means the bond looks towards you
sits in front of the paper plane
dotted triangles: means the bond looks away from you
sits behind the paper plane
cis-trans isomers
Depending on whether the substituents of highest priority at each end of the double bond are next to or opposite each other
Occurs with alkenes
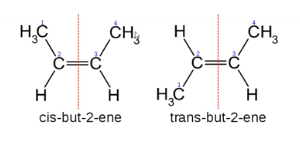

E and Z isomers
the E, Z system must be used for tri- tetra-substituted alkenes (3 or 4 different bonded groups)
To assign an E, Z configuration, first assign a priority to the substituents on each side of the double bond
Priority based on atomic number
Highest atomic number= highest priority
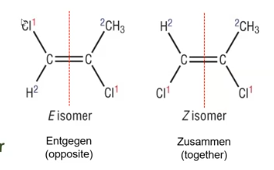
number 1 group on opposite sides or same sides

chirality
when a molecule is not identical to its reflection
mirror image is not superimposable with itself
achirality
when a molecule is identical to its reflection
superimposable
have a plane of symmetry (an imaginary plane passing through an object dividing it such that one half is the mirror image of the other half)
chiral center
a carbon atom that is bonded to 4 different groups
stereogenic center
induces chirality
asymmetric carbon center
stereoisomers; enantiomers
direct mirror images of a molecule/pairs of mirror images of chiral molecules
do not differ in physical characteristics
interact differently with polarised light and with stereospecific environments (e.g. active sites, receptors/proteins on our tongue are chiral environments that can perceive the different enantiomers)
one enantiomer will shift the light to one side of the plane, and vice versa
stereoisomers; naming enantiomers
use the Cahn, Ingold, Prelog systems of nomenclature
used to designate configuration at a stereocenter (chiral center)
labels R and S are used to differentiate between enantiomers
priority of the 4 different substituents is assigned through atomic number; the next connected atom to the C is used for atomic priority numbering
steps for naming enantiomers
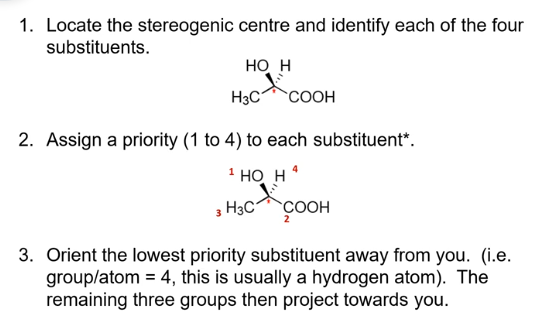
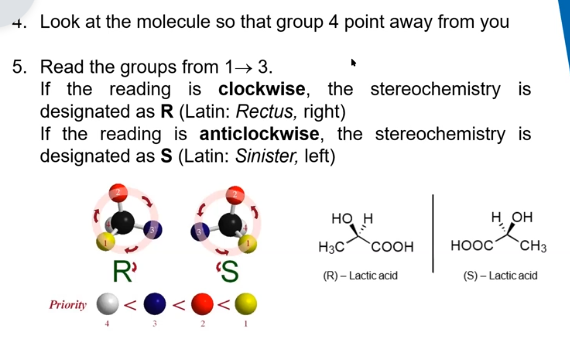

stereocentres and stereoisomers
1 stereocentre, 21=2 stereoisomers are possible
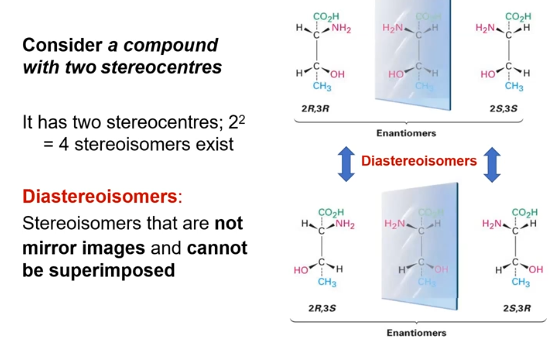
2 stereocentres, 22=4 stereoisomers are possible
general rule: for a molecule with n stereocentres, a max of 2n are possible
diastereoisomers
stereosiomers that are not mirror images and cannot be superimposed

meso compounds
Tartaric acid has two chiral centres
Therefore has 4 stereoisomers that exist
Two of them are enantiomers
The other enantiomers actually create an achiral molecule with an internal symmetry plane within the molecule
Thus these are not enantiomers
THIS IS CALLED THE MESOFORM
enzymes
chiral substances which only produce or react with substances that match their stereochemical requirements