gen chem 2 vocab and equations
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
immiscible
Describes liquids that do not mix or blend together
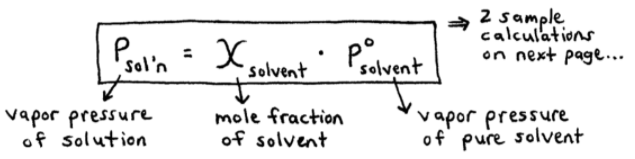
Raoult’s Law
used for vapor pressure based on concentrations (mole fraction)
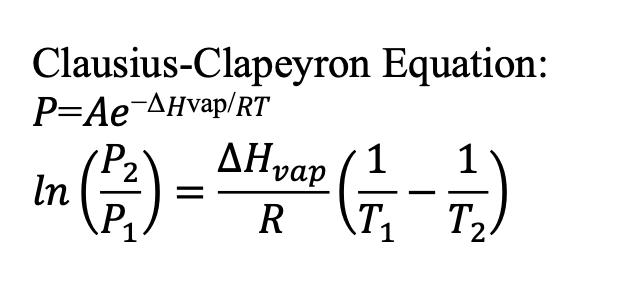
Clausius-Clapeyron Equation
connects vapor pressure, temperature, and the enthalpy of vaporization
miscible
liquids of similar polarities that mix together
k units - zeroth order
concentration/time or M/s
k units - first order
1/s or s-1
k units - second order
1/(M·s) or M-1s-1
when Q=0,
the reaction has not progressed
when Q>K,
the reaction shifts to the left, goes reverses, makes reactants
when Q<K
reaction goes forward, shifts to the right, makes products
when Q=K
reaction is at equilibrium
1st law of thermodynamics
energy cannot be created nor destroyed
2nd law of thermodynamics
for a spontaneous process, the entropy of the universe will always increase, dSuniverse > 0, dSsystem+dSsurroundings > 0
3rd law of thermodynamics
entropy of a perfect crystal at absolute zero (0 K) is zero
the common ion effect
when a substance with a similar ion as a reactant is added to a solution where a similar ion exists, causes a reverse shift in equilibrium and a decrease in solubility
when dG > 0
the reaction is non-spontaneous
when dG < 0
the reaction is spontaneous
cathode
where reduction occurs
anode
where oxidation occurs
the oxidizing agent is:
the oxidant, being reduced
the oxidant is:
the oxidizing agent, being reduced
the element being reduced is:
the oxidant, the oxidizing agent
the reductant is:
the reducing agent, being oxidized
the reducing agent is:
being oxidized, the reductant
the element being reduced is:
the oxidant, the oxidizing agent
positive dG means
the reaction is non spontaneous
a negative dG means:
the reaction is spontaneous
when dS and dH are both negative,
the reaction will be spontaneous at low temperatures and non-spontaneous at high temperatures
when dS and dH are positive,
the reaction will be non-spontaneous at low temperatures and spontaneous at high temperatures
when dS is positive and dH is negative,
the reaction will be spontaneous
when dS is negative and dH is positive
the reaction will ne non-spontaneous