chapter 2 - the chemical basis of life
1/82
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
83 Terms
matter
anything that occupies space and has mass
found on earth in three physical states: solid, liquid, and gas
*living organisms are made of matter
element
a substance that cannot be broken down into other substances by ordinary chemical means
each element has a symbol made up of the first letter or two of its english, latin, german, name (O - oxygen)
*types of matter are all composed of elements
compound
a substance consisting of two or more different elements combined in a fixed ratio
compounds have different characteristics than the elements that make up the compound
ex. H and O (gases) -→ H20 (water)
*key idea of emergent properties
major elements essential for life
*first six elements make up 99% of the human body
oxygen (O): 65%
carbon (C): 18.5%
hydrogen (H): 9.6%
nitrogen (N): 3.3%
calcium (Ca): 1.5%
phosphorus (P): 1.0%
potassium (K): 0.4%
sulfur (S): 0.3%
sodium (Na): 0.2%
chlorine (Cl): 0.2%
magnesium (Mg): 0.1%
the four major elements of biological molecules
oxygen (O)
carbon (C)
hydrogen (H)
nitrogen (N)
oxygen (O)
atomic number: 8
when neutrally charged, it has 8 electrons (6 in its valence shell)
*can make TWO bonds
electronegativity: 3.44
highest of the four main elements
carbon (C)
atomic number: 6
when neutrally charged, it has 6 electrons (4 in its valence shell)
*can make FOUR bonds - vital in making organic compounds
electronegativity: 2.55
similar to that of hydrogen (forms nonpolar covalent bonds with H)
hydrogen (H)
atomic number: 1
when neutrally charged, it has 1 electron (1 in its valence shell)
*can make ONE bond
electronegativity: 2.2
similar to that of carbon (forms nonpolar covalent bonds with C)
nitrogen (N)
atomic number: 7
when neutrally charged, it has 7 electrons (5 in its valence shell)
*can make THREE bonds
electronegativity: 3.04
elements in which are components for bone and teeth
calcium (Ca)
phosphorus (P)
most of elements in the remaining 1% of the body
*these elements are involved in functions such as nerve signalling and chemical reactions (redox)
potassium (K)
sulfur (S)
sodium (Na)
chlorine (Cl)
magnesium (Mg)
trace elements
present elements in minute quantities, making up less than 0.01% of the human body
baron, chromium, cobalt, copper, fluorine, iodine, iron, manganese, molybdenum, selenium, silicon, tin, vanadium, and zinc
some elements are required by some organisms but not all
iodine (I) is for organisms only with a vertebrate (a backbone)
ex. iron (Fe) is a trace element needed by all forms of life (vital for energy processing and transporting oxygen into the blood) — 0.004%
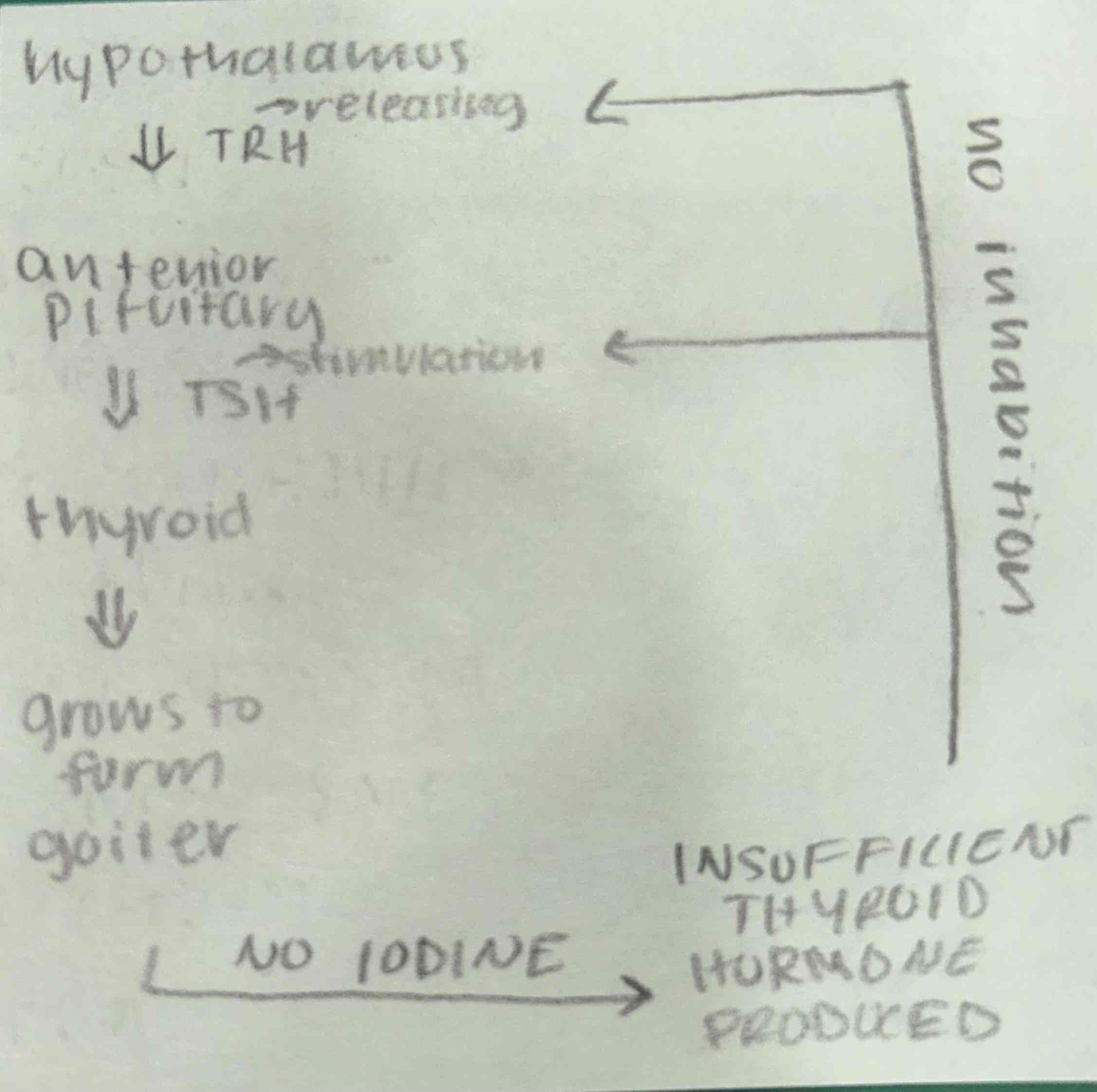
iodine as a trace element
iodine is an essential component of a hormone produced by the thyroid gland
a deficiency in the diet causes the thyroid gland to grow to an abnormal size (condition is called a goiter)
serious effects take place during fetal development and childhood
miscarriages, poor growth, mental impairment
seafood, kelp, dairy, and leafy greens are great sources
inland regions are common for iodine deficiencies
*universal consumption of iodization of all salt (iodized salt)
iron as a trace element
iron deficiency is the most common nutritional disorder with two billion people affected (mainly children and women)
to avoid, food fortification, iron supplements, and diet diversification/improvement
however, too much iron could be fatal and could damage organs and increase risk of disease
fluoride as a trace element
fluoride, a form of fluorine, helps with maintaining strong bones and public health
is added to our water (government controls this —> debates whether or not they should have the right too or not)
fluoride in toothpaste helps maintain integrity of the tooth and prevent cavities
too much makes the teeth look brown, but significantly reduces the amount of cavities
atom
the smallest unit of matter that still retains the properties of an element
each element has its own type of atom, which is different from the atoms of other elements
subatomic particles
parts of an atom
proton
electron
neutron
proton
a subatomic particle with a single positive electrical charge (+)
the amount of proton determines the element of the atom
electron
a subatomic particle with a single negative electrical charge (-)
the distribution if electrons determines an atom’s chemical properties
only electrons are directly involved in the chemical activity of an atom
neutron
a subatomic particle that is electrically neutral (no charge)
the amount of neutrons determines the stability of the atom and forms different isotopes.
nucleus
the atom’s central core
made up of protons and neutrons
electron cloud
formed by negative charge by the two electrons surrounding the nucleus
attraction between the electrons and protons holds the electrons near the nucleus
atomic number
how many protons an atom has
the amount of protons an atom has determine the element
*unless indicated, an atom has an equal number of protons and electrons (net charge = 0)
atomic mass
is approximately equal to its mass number - the sun and its protons and neutrons - in daltons (commonly an average of isotopes)
radioactive isotopes with basic research
used carbon dioxide (CO2) containing the radio isotope, carbon-14, to study photosynthesis
allowed researchers to trace the sequence of molecules made by plants in the chemical route from CO2 to sugar
radioactive isotopes with medical diagnosis and treatment
used to tag chemicals that accumulate in specific areas of the body (ex. phosphorus in bones)
scans and camericas can show where the radiation collects
using radioactive iodine to kill the cancer in the thyroid as it usually accumulates there
PET (positron-emission technology) scan, can produce images of areas of the body with high metabolic activity (with using glucose/oxygen that is radioactive)
*extremely helpful in diagnosing heart disorders, cancers, and aiding in brain research such as alzheimer’s
dangers of radioactive isotopes
particles and energy thrown off my radioactive atoms can damage molecules and DNA
explosion of a nuclear reactor in chernobyl (ukraine - 1986)
radon (a radioactive gas) is the second leading cause of cancer
electron shells
locations in which an electron could be, each with a characteristic distance from the nucleus
depending on an atom’s atomic number, an atom may have one, two, or more electron shells
*the first shell holds TWO electrons, the second shell holds EIGHT electrons, the third shell holds EIGHTEEN electrons
orbitals
discrete volumes of space in which electrons are most likely to be found
within each shell, electrons travel in different orbitals
each orbital can hold a maximum of TWO electrons (ex. the 2nd shell has four orbitals and can hold up to eight electrons)
valence shell
the outermost electron shell of an atom
the amount of electrons in that shell mostly determines the chemical property of an atom
atoms whose outer shells are not full tend to interact with other atoms in a way that enable them to complete or fill their valence shells (if the valence shell is full, they will rarely react with other atoms — usually classified as noble gases)
*hydrogen, oxygen, nitrogen, and carbon react with other atoms to form bonds
valence/bonding capacity
the number of covalent bonds an atom can form depends on the number of electrons needed to fill its valence shell
hydrogen needs 1
oxygen needs 2
nitrogen needs 3
carbon needs 4
chemical bonds
attractions in which holds atoms together due to their interactions (giving up, accepting, or sharing electrons)
ionic bonds: the transfer of electrons between atoms result in this bond
covalent bonds: the sharing of electrons result in this bond
covalent bonds
joins atoms into molecules through electron sharing
molecule
consists of two or more atoms held together by covalent bonds
the sharing of electrons is not always equal
electronegativity
a measure of attraction between atoms
a low difference in electronegativity result in nonpolar covalent bonds
a medium difference in electronegativity result in polar covalent bonds
a large difference in electronegativity result in ionic bonds
polarity
refers to a separation of charges
think of the north and south poles of earth, or a magnet
nonpolar covalent bonds
the electrons are shared EQUALLY between two atoms of the same of similar electronegativity
polar covalent bonds
the electrons are drawn more closely to the more electronegative element between the two atoms that differ in electronegativity
as a result, the more electronegative atom carries a partially negative charge in the molecule and the other carries a partially positive charge in the molecule
molecular formula
shows the number of atoms of each element using symbols and subscripts
electron distribution diagram
shows how each atom completes its outer shell by sharing one or more pairs of electrons
structural formula
shows a molecule’s approximate shape and represents each covalent bond with a line
one line represents a single bond
two lines represents a double bond
space-filling model
uses a color-coded ball for each atom and comes closest to representing a molecule’s 3-D shape
ionic bonds
attractions between ions of opposite charge
two atoms are so unequal in their attraction for electrons that the more electronegative atom strips an electron completely from another atom
ex. NaCl (table salt) — Na gives an electron to Cl to complete their valence shells in which their resulting charges form a bond
redox reaction
a chemical reaction in which involves a transfer of electrons between two atoms
the transfer of an electron moves one unit of negative charge from one atom to another
reduction: when an atom loses an electron (becomes positively charged) — ex. Na
oxidization: when an atom gains an electron (becomes negatively charged) - ex. Cl
ions
an atom or molecule with an electrical charge resulting from a gain or a loss of one or more electrons
when the attraction between two ions with opposite charges holds them together is an ionic bond
cation: positively charged ion
anion: negatively charged ion (names of anions often end in “-ide”)
salts
a synonym for an ionic compound
affects of the environment on ionic bonds
when DRY, bonds are so strong you need a hammer and a chisel to break the crystal
when IN WATER, ions interact with polar water molecules and the salt dissolves
strong and weak bonds
covalent bonds are strong, linking atoms to a cell’s molecules
ionic bonds are weaker, but crucial to the functioning of a cell concerning bonds within and between molecules
hydrogen bonds are an important week bond between atoms and molecules consisting of hydrogen
hydrogen bonds
an electrostatic force of attraction, “flirtation,” between a partial positive change that allows each hydrogen to be attracted to a nearby atom with a partially negative charge (oxygen in a water molecule for example)
called hydrogen bonds because one atom in this attraction is always an hydrogen bond
*usually represented by dotted lines
polar molecule
one or more sides are slightly positively charged and the other is slightly negatively charged
unequal distribution of charges
polar molecules adjust or turn when in a magnetic field so that the side of the molecule with the positive charges are facing or attracted towards the plate with negative charges as it experiences an electric force in the direction of the field (and vice versa)
nonpolar molecule
the charges are distributed evenly throughout the molecule
no “poles”
nonpolar molecules do not experience a force when in a magnetic field since their charges are evenly distribute throughout
intermolecular forces
attractions between molecules
what holds molecules together
dipole-induced dipole
an intermolecular force that occurs between polar molecules and non polar molecules
acts between a polar molecule and a nonpolar molecule as the dipoles of the polar molecule “induce” a temporary dipole in a nonpolar molecule which forms an attraction
london dispersion
an intermolecular force that is inevitable in attractions between any molecule, both polar and nonpolar (however, is the only intermolecular force between nonpolar molecules)
acts between nonpolar molecules as they form temporary dipoles and become temporarily polar to form an attraction
*because london dispersion does not rely on polarity, its strength is due to the size and shape of the molecules
chemical reactions
breaking chemical bonds and forming new ones
matter cannot be created or destroyed, they can only be rearranged
*our cells are constantly rearranging molecules
reactants
conversion of starting molecules in a chemical reaction
product
the material resulting from the chemical reaction
cohesion
the tendency of molecules of the same kind to stick together
stronger for water than most other liquids due to hydrogen bonds between water molecules (though they only last for a few trillionths of a second)
ex. paper clip floating on top of the water versus sinking in
the water bonds together through hydrogen bonding which creates a film that holds up the paper clip
highly important in the living world
trees depend on cohesion to help transport water and nutrients from the roots to the leaves
surface tension
a measure of how difficult it is to stretch or break the surface of a liquid (for water, due to its hydrogen bonds)
an extension of the concept on cohesion
adhesion
the clinging of one substance to another
stronger for water than most other liquids due to hydrogen bonds between water molecules and other polar substances (though they only last for a few trillionths of a second)
ex. water travelling more up a capillary tube than alcohol despite gravity
water molecules are more polar than alcohol molecules and they form hydrogen bonds with other polar molecules (the glass in this case), resulting in a stronger attraction and climbing of water molecules up the tube
in plants, the thinness of a plant’s veins enhances the adhesion of water to its cell walls (and counters the force of gravity)
thermal energy
the energy associated with the random movement of atoms and molecules
heat
thermal energy in transfer from a warmer to a colder body of matter
temperature
a measure of intensity of heat
the average speed of molecules in a body of matter
because of hydrogen bonding, water has a stronger resistance to temperature change than most other substances
high specific heat capacity
resistance to temperature change (due to hydrogen bonding for water)
heat must be absorbed to break hydrogen bonds
to raise the temperature of water, hydrogen bonds between molecules must be broken before the molecules can move faster
water absorbs a large amount of heat, most of it disrupting hydrogen bonds, while warming up only a little
ex. warming up water and oil
the rate of temperature increasing for water is SLOWER than that of oil which is faster than water
water has a high heat capacity compared to oil, which means that water can take in more heat before the temperature of the substance increases
high specific heat of evaporation
when a substance evaporates (changing its physical state from a liquid to gas) and the surface of the liquid that remains cools down
occurs because the molecules with the greatest energy (the hottest ones) leave
heat is released when hydrogen bonds forms
when water cools, water molecules slow down and more hydrogen bonds form, releasing a considerable amount of heat
ex. alcohol evaporating faster than water
water has a higher specific heat of evaporation as it requires more heat to increase the thermal energy to evaporate than alcohol
density
ice floats because it is less dense than water
as water freezes, each molecule forms stable hydrogen bonds with other water molecules in a certain formation and creating a -D crystal
formation forces the atoms to be more spaciously apart in ice whereas in water, molecules are more tightly packed (concentration of mass in volume)
ex. ice floats on oil in which forms a layer between ice and water, and when ice melts, the water droplets sinks to the bottom
ice is less dense than water because of the arrangement of molecules with hydrogen bonds allow for it to be less packed with mass per volume than water which is more packed since hydrogen bonds are constantly breaking and reforming
if ice were to be more dense: ponds, lakes, and oceans would freeze
*when water cools, ice serves as an insulating blanket that traps heat in water and prevents it from freezing (keeps marine life alive and serves as a ground for polar bears)
solution
a liquid consisting of an uniform mixture of two or more substances
solvant
the dissolving agent
aqueous
when water is the solvant
solute
a substance that is being or is dissolved
water as a solvant
water is the solvant of life which its versatility as a solvant results from the polarity of its molecules
can dissolve both ionic compounds and polar molecules
ionic compounds in water
when ionic compounds dissolve in water
the positively charged hydrogen ends of the water molecules form attractions to the negatively charged ends of the ionic compound
the negatively charged oxygen ends of the water molecules cling to the positively charged ends of the ionic compound
these processes work inward from the surface of the ionic compound in which water molecules eventually surrounded and separate all ions
ex. table salt — NaCl
polar molecules in water
when polar molecules dissolve in water:
the positively charged hydrogen ends of water molecules are attracted to the negatively charged ends of the polar molecules
the negatively charged oxygen ends of water molecules are attracted to the positively charged ends of the polar molecules
these processes continue until all water molecules surround the compound and form hydrogen bonds with its polar regions
ex. sugar
dissociation of water molecules
water molecules breaking into hydrogen ions (H+) and hydroxide ions (OH-)
in liquid water, a very small percentage of water molecules break apart into ions
however, these ions are highly reactive
changes in concentration can drastically affect a cell’s proteins and other complex molecules
some chemical compounds help add or remove H+ from an aqueous solution
acid
a substance that donates hydrogen ions to solutions
has a higher concentration of H+ than OH-
ex. hydrochloric acid (HCl) is an acid in the gastric juice of the stomach
base
a substance that reduces the hydrogen ion concentration in a solution
some bases like sodium hydroxide (NaOH) donates OH, which forms with H+ to form water molecules, thus reducing the concentration
this is a common ingredient in oven cleaners
other bases accept H+ from a solution, increasing -OH concentration
pH scale
describes how acidic or basic a solution is (pH means potential for hydrogen)
ph 0 is the most acidic to pH 14 which is the most basic
decrease in pH equals increase in acidity (more H+ concentration)
increase in pH equals increase in basicity (more OH- concentration)
each pH unit represents a 10-fold change in the H+ concentration of a solution
lemon juice at pH 2 has 10x more H+ than cola at pH3 and has 100x more H+ than tomato juice at pH4 (pH 7 is 10^-7 mol H+)
neutral pH
pH of 7
pure water and aqueous solutions that are not acidic not basic are neutral
concentration of H+ and OH- are equal
*the pH of most cells is close to 7 and blood pH is close to 7.4
a person cannot survive for more than a few minutes if blood pH drops to 7.0 or rises to 7.8
buffers
substances that minimize changes in pH
in biological fluids to maintain homeostasis
accepts H+ when in excess or donates when depleted
carbon dioxide
main product of fossil fuel combustion
the increasing the release of CO2 into the atmosphere is what causes climate change
25% is absorbed by oceans (which seems like a good thing at first) but levels are rising and the increased absorption is harming marine life and ecosystems
ocean acidification
CO2 dissolving in seawaters lowers the pH of ocean water
impact of lower pH on coral reefs
as seawater acidifies, extra H+ combine with carbonate ions (CO32-) to form bicarbonate ions (HCO3-)
*this reduces the carbonate ion concentration available to corals and other shell-building animals for calcification (lower the concentration of carbonate ions, lower the rate of calcification, thus slows the growth of corals and animals)
calcification
coral animals combine calcium and carbonate ions to form their calcium carbonate skeletons
scientist research on ocean acidification
controlled experiment (artificial habitat)
constant variables are the pH, temperature, calcium ions concentration
varied variable is the carbonate ion concentration in sea water
*provided evidence that ocean acidification and its resulting reduction in carbonate ion concentration negatively affects coral reefs
observation or study (natural habitat)
carbon dioxide is being released by underground volcanos which lowers the pH of seawater
*reduction in coral diversity and shifts to a lesser structurally complex and slower growing coral reefs
scientists synthesize conclusions using multiple lines of evidence
ocean acidification results from both where the pH naturally varies which has an impact on the health of coral reefs and the diversity of organisms they suppitt
a decrease in structural integrity equals a decrease in organisms that they can support (coral reeds are havens of organism diversity)
presence of water in the search of extraterrestrial life
the presence of water is important in the search for extraterrestrial life
emergent properties of water supports life on earth (idea if life was able to form or exist in environments with water)
water plays important roles from moderating temperature to functioning as the solvant of life
ex. mars
has ice caps at both pole and has signs that water may exist elsewhere on the planet