Formation of ions
1/7
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
What are ions?
Charged particles
How to atoms try gain full outer shells?
Through losing or gaining electrons
What happens when metals form ions?
they lose electrons to form positive ions
What happens when a non-metals forms ions?
gain electrons to form negative ions
What is the charge of any ion?
The electrons it gained or lost
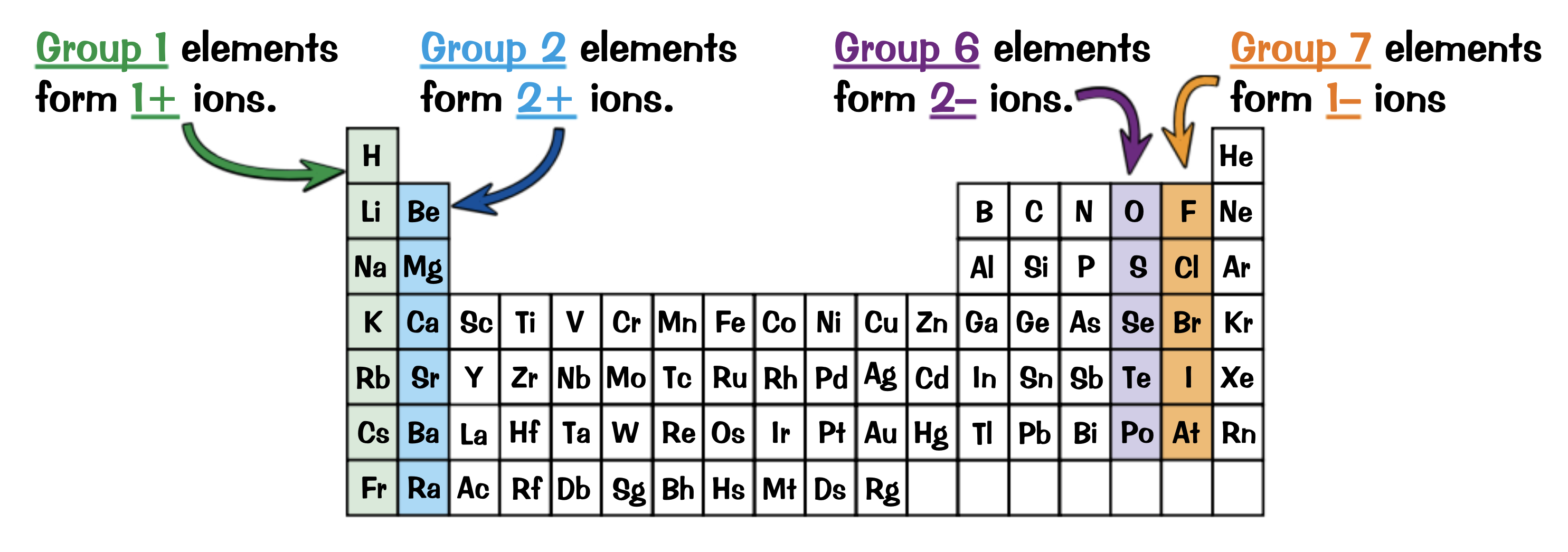
The elements in which groups are most likely to form ions?
1,2,6,7
How do you find an ions charged for an element?
Their group number is how many electrons they have in their outer shell, so they will all need to gain or lose the same number of electrons
Write the ions each group will form and a half equation for an element in each
Group 1: 1+ ions ( Na→ Na+ + e- )
Group 2: 2+ ions ( Mg → Mg2+ + 2e-)
Group 6: 2- ions ( Cl + e- → Cl- )
Group 7: 1- ions ( O + 2e- → O2- )