Chemistry Topic 1
5.0(1)
Card Sorting
1/22
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
1
New cards
Define Avagadro’s constant
The nuber of atoms of carbon in 12.00g of C-12.
2
New cards
Relative molecular mass units
none
3
New cards
Molar mass units
gmol^-1
4
New cards
Define the Empirical Formula
the simplest whole number ratio of the elements in a compound
5
New cards
Define the Molecular Formula
a whole-number multiple of the empirical formula of a compound
6
New cards
What happens to the number of moles and concentration when something is diluted
Number of moles stays the same, volume is increased, concentration decreases
7
New cards
Define concentration
a measure of the amount of solute ina specified volume of a solvent
8
New cards
Define limiting reagent
The reactant that determines the amount of product that can be formed (other reactants are said to be in __**excess**__)
9
New cards
Calculate percentage yield
(actual/theoretical) x 100 %
10
New cards
Define Standard Solution
A solution with an accurately known concentration
11
New cards
Define Primary Standard
Substances that are so pure that the no. of mol can be accurately calculated from their mass
12
New cards
Define the equivalence point
The point in titration when the chemicals mixed have reacted in the mole ratio indicated by the balanced chemical equation
13
New cards
Define solution
a homogenous mixture of a solute and a solvent
14
New cards
Diagram of homogenous and heterogenous solution
**Heterogeneous solutions are those that have non-uniform compositions and properties throughout the solution.**
\
Homogeneous solutions are those that have the same composition and properties throughout the solution.
\
Homogeneous solutions are those that have the same composition and properties throughout the solution.
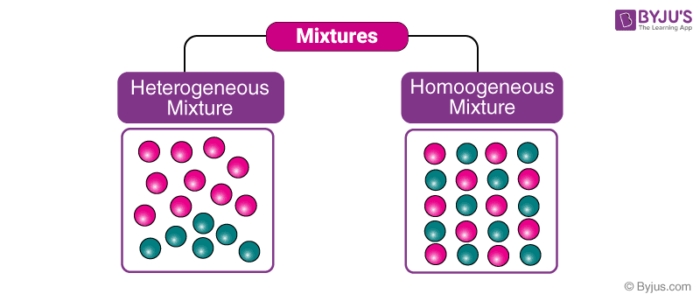
15
New cards
Boyle’s Law
Pressure is inversely proportional to Volume at a constant temperature
16
New cards
Charle’s Law
Volume is proportional to temperature at a constant pressure
17
New cards
Gay Lussac’s Law
Pressure is proportional to temperature at a constant volume
18
New cards
When is a gas ideal
* particles are completely independent
* volume of individual gas particles is negligible
* PV/RT = 1
* volume of individual gas particles is negligible
* PV/RT = 1
19
New cards
Avogadro’s Law
Equal volumes of gases at the same temperature and pressure contain equal numbers of particles. (Volume is proportional to no. of mol)
20
New cards
Molar volume at STP
22\.7dm^3
21
New cards
dm^3 with
kPa
22
New cards
m^3 with
Pa
23
New cards
When do real gases behave the most ideal
High Temperature, Low pressure