2. Proteins
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
74 Terms
Sources of proteins
Animal proteins
Milk proteins
Egg (white) proteins
Animal by-products (blood/gelating)
Plant proteins
Soy proteins
Pea proteins
Lupin proteins
Sunflower proteins
Novel (alternative) proteins
Algae proteins
‘leafy’ proteins
Microbial proteins
Function of proteins in nature
Nutrition: source of nitrogen and essential maino acids
Structure: (collagen → gelatine)
Metabolism: Homeostasis, hormones, enzymes, antibodies
Function of proteins in food
Nutrition: meat, cheese, nuts
Texture: gluten in bread, gelatin in sweets
Taste: Maillard reaction
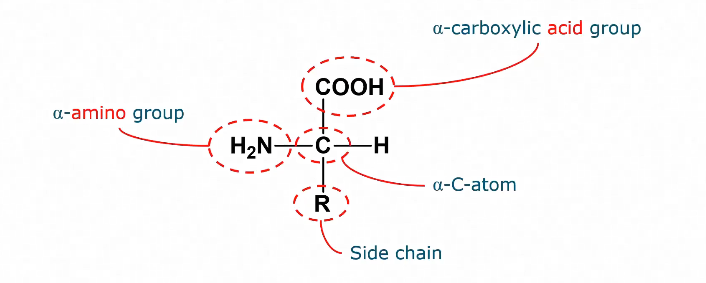
Structure of amino acids
Contains an amino group
And a carboxylic acid group
Contain a different side chain
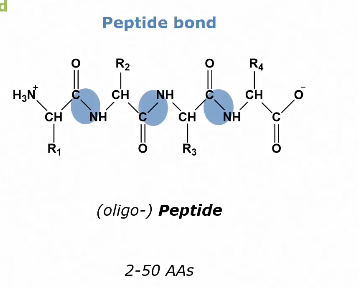
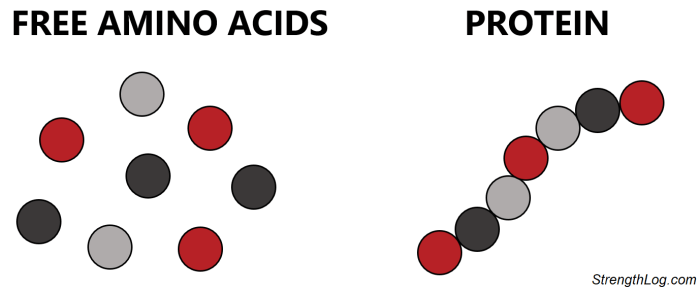
How are amino acids bonded together?
Via peptide bonds
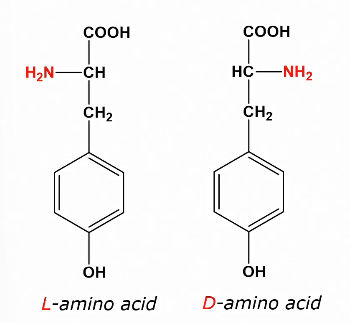
L and D amino acids
As amino acids are chiral, they can have the same molecular formula but a different structure.
The L variant of amino acids occurs naturally
During processing, amino acids can turn into D amino acids which are not digested well.
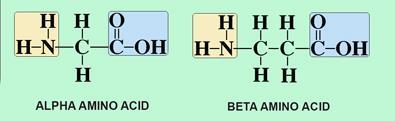
Alpha and beta amino acids
Alpha amino acids are common and beta amino acids only exist in plants.
Beta amino acids do not naturally occur in proteins
Polar charged amino acids
When an amino acid has a carboxyl or amino group
This causes different properties.
Hydrophilic
High solubility
Mainly present on the outside of the protein
Reactive
Charge depends on pH
Polar non charged amino acids
Contain a side chain with: a hydroxyl group, a sulfhydryl group or an amide group
Properties:
Hydrophilic
High solubility
Mainly present on the outside of the protein
Reactive
Non-polar non-charged amino acids
Contains aliphatic group, aromatic group, imino acid or thio-ether, CH3
Properties:
Hydrophobic
Low solubility
Mainly present on the inside of the protein
Occurrence of peptides
In nature:
Blood (buffering function)
Plants (hormones)
In food:
Bread (glutathione: breaks down gluten and helps to retain bread airyness)
In foods after hydrolysis:
Microbial: fermentation products
Enzymatic: protein hydrolysates
Function of peptides
Are more reactive than proteins
Can have taste (often bitter)
Aspartame: a very sweet peptide
Are more easily taken up by the body
Are studied for their bio-functional properties
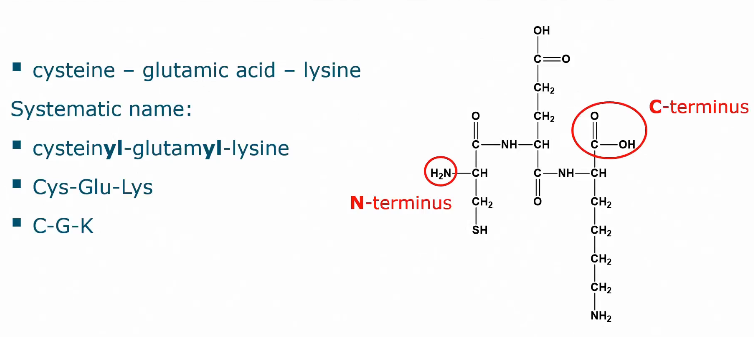
Number of amino acids in a peptide naming

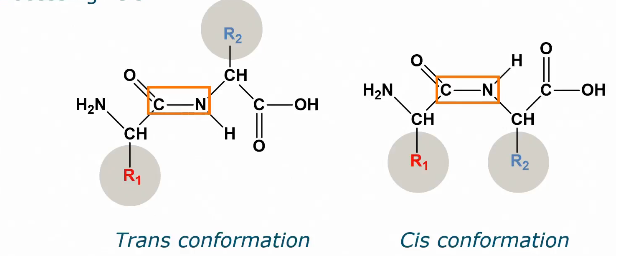
Two different peptide bonds
Naturally: Trans (side chains do not hinder each other, energetically favorable)
Only after processing: cis
Nomenclature for peptides
When the amino acid is not bonded via a peptide bond to the alpha carbon, then a gamma is written before the name.
What changes the protein structure?
Temperature
pH and ionic strength
Solvent
Results in changes in:
Solubility
Functionality
Structural forces in proteins
Covalent bonds (atoms are really connected)
Not bonds but interactions:
Hydrogen bonds
Hydrophobic interaction
Electrostatic interaction
What influences the structural forces in proteins?
pH and isoelectric point
Salt
Temperature
Solvent (water, ethanol)
Primary structure
Sequence of amino acids
Secondary structure
Known as alpha helix and beta sheet
Alpha helix:
Stabilized by hydrogen bonds
Beta sheets:
Stabilized by hydrogen bonds
Tertiary structure
Stabilized by:
Electrostatic and hydrophobic interactions
Disulfide bonds
Van de Waals interactions
Quaternary structure
Spatial arrangement of different polypeptide chains
Stabilized by:
Electrostatic and hydrophobic interactions
Disulfide bonds
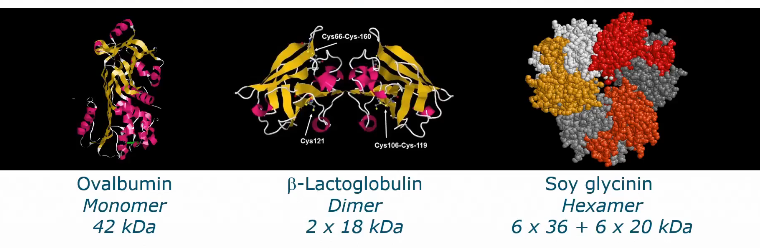
Classification of food proteins
Protein structure
Important for solubility and functionality
Protein solubility
Important for food applications
Different proteins and their solubility
Globular proteins (milk, blood and soy): high solubility
Combination of a helices and B sheets alternated by random coils
Fibrillar proteins (gelatin, meat): low solubility
The entire peptide is arranged within a single regular secondary structure
Random coil proteins (caseins): high solubility
Can turn into micelles
Other proteins (gluten): low solubility
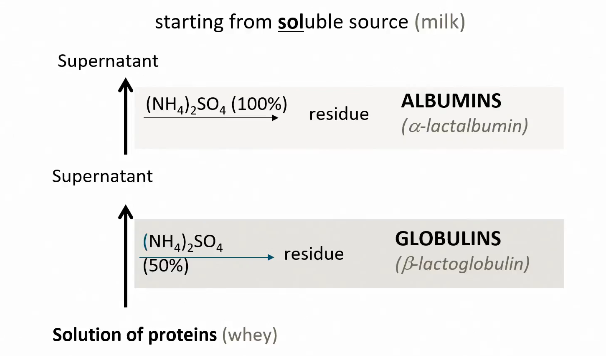
Classification based on solubility
Osborne fractionation
Can help to identify different amino acids in protein.
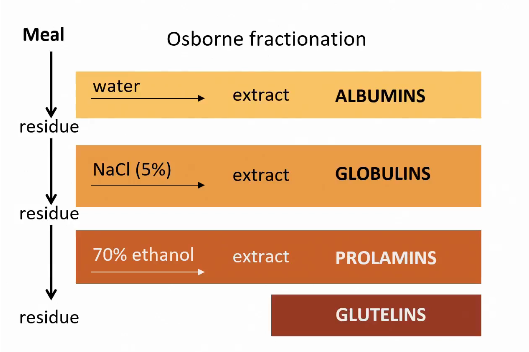
Classification based on in-solubility
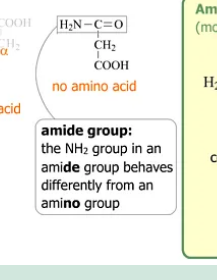
Amide group
Is not an amino acid!!
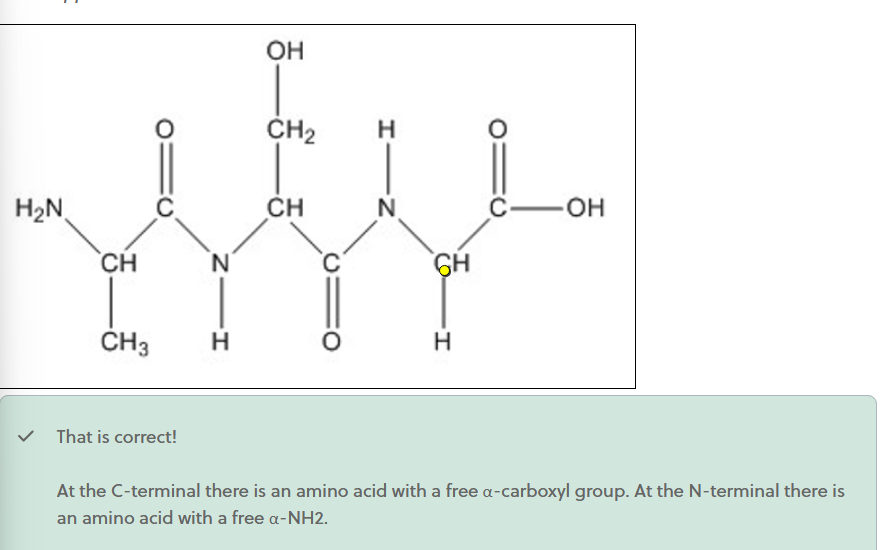
Where is the C-terminal?
Electrostatic interactions between proteins
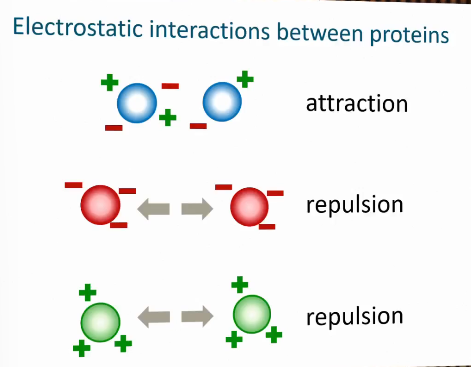
Consequence of charge for functional properties
At the isoelectric point proteins are slightly attracted to each other due to hydrophobic forces
Can aggregate
Solubility is low at isoelectric point.
Charged proteins can have interactions with other charged molecules.
Isoelectric point
The pH at which a molecule, such as a protein or amino acid, has no net electrical charge
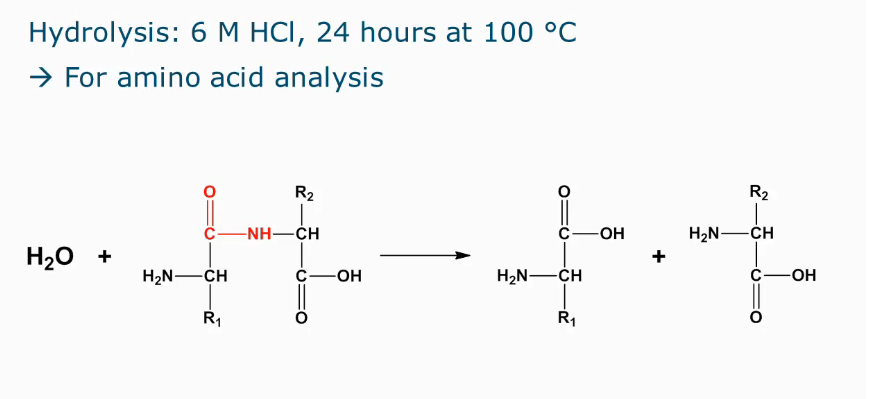
Chemical reactions in proteins during processing
Hydrolysis of peptide bonds
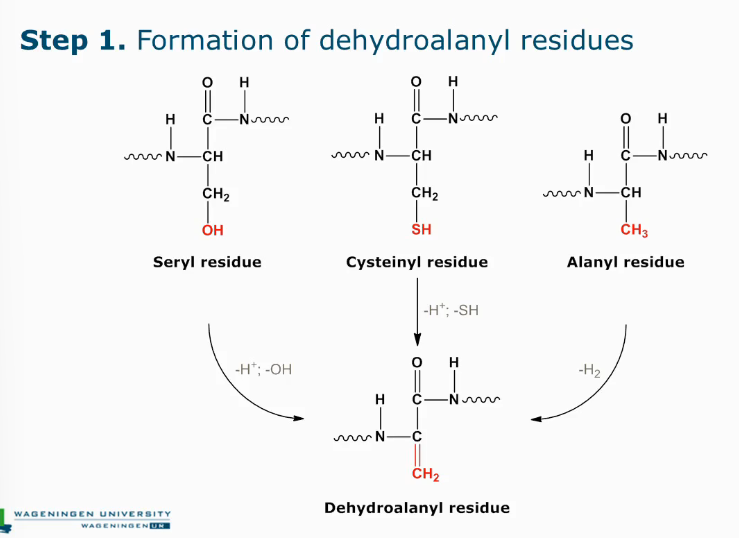
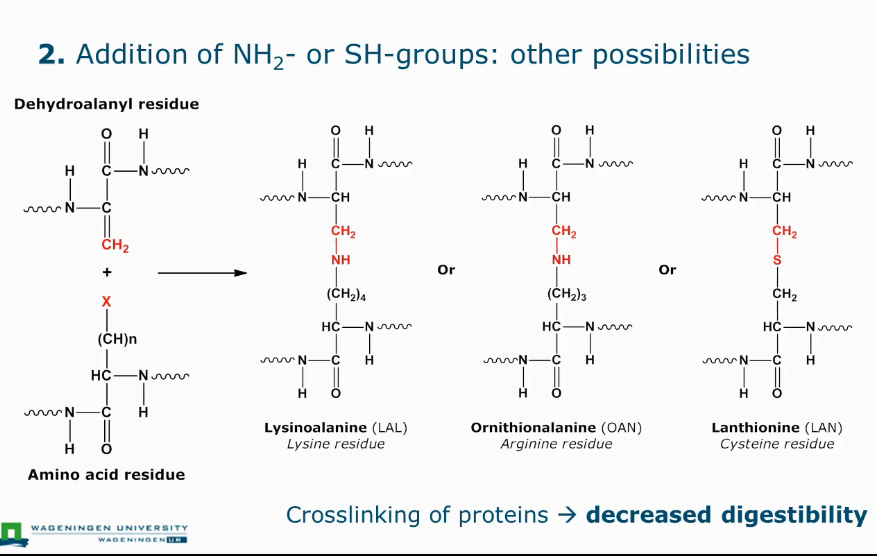
Formation of lysino-alanyl derivatives
Formation or reshuffling of S-S bridges
Deamination of decarboxylation
Maillard reaction (works better at extreme high pH or extreme low)
Effect of processing proteins
Loss of essential amino acids
Crosslinking of proteins → decreased digestibility
Change in texture
Change of allergenic potential
Formation of aroma compounds/off-flavors
Change of charge
Hydrolysis of peptide bonds
Lysino-analyl derivatives step 1
Lysino-analyl derivatives step 2
Lysino analnyl derivatives and boiling
The more boiling, the more lysinoalanine is formed
Therefore the derivatives are an indication of heat damage
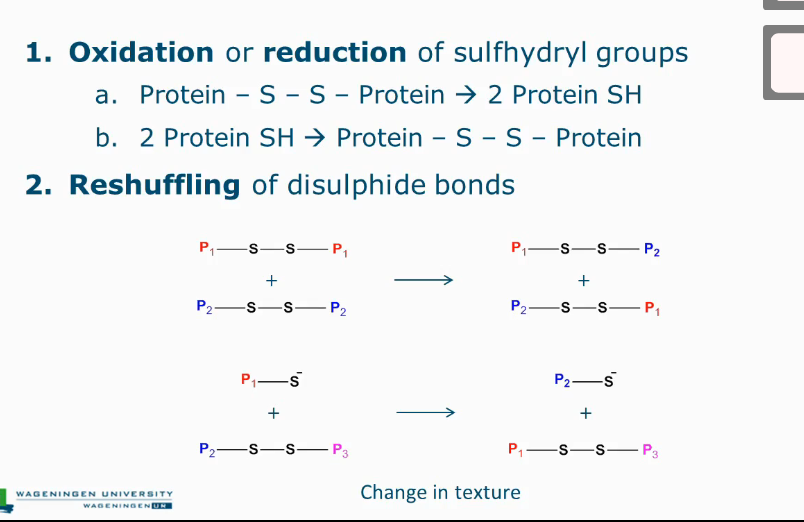
Reactions with disulfide bridges
Oxidation or reduction of sulfhydryl groups
Reshuffling of disulphide bonds
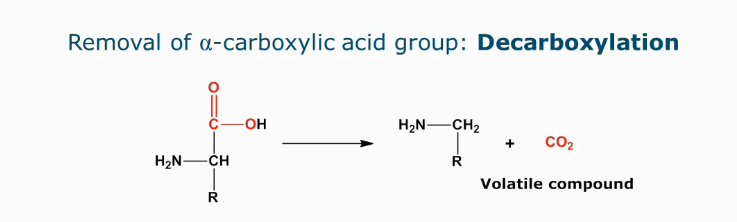
Decarboxylation
Indicator of microbial spoilage as volatile compounds are formed (aroma and off-flavors)
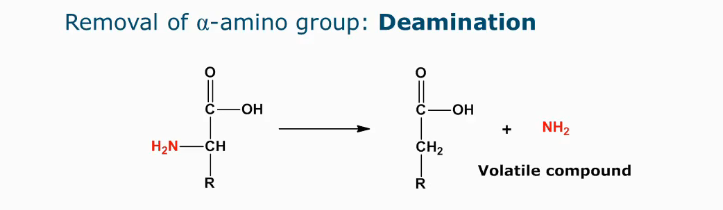
Deamination
Indicator of microbial spoilage as volatile compounds are formed (aroma and off-flavors)
Side group needs to have an OH on the beta carbon atom (if its enzymatic, de-amination is possible for all amino acids)
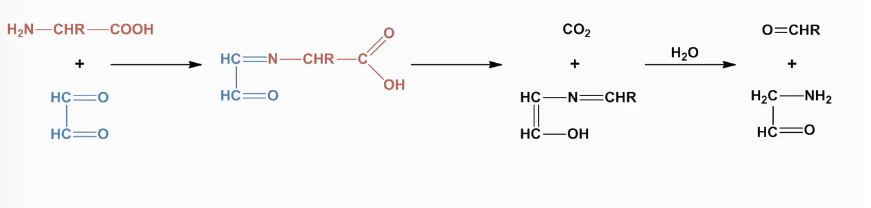
Strecker degradation
combination of decarboxylation and deamination
Removal of a-carboxylic acid and a amino gorup:
results in:
Formation of aldehydes (aroma compounds)
Free amino acids vs amino acids
Requirements for an isopeptide bond formation
The side group needs to contain a -COOH
Disadvantage of reactions that can cause crosslinking op peptides
Can decrease digestibility
Disadvantage of amino acids with reactive side groups
Can cause loss of essential amino acids
Physical changes caused by heat
When heated above denaturation temperature (60-80C)
At timescales of seconds - minutes
Increased mobility of protein molecule
Stress on the stabilizing interactions
Exposure of hidden hydrophobic groups
Exposure of S-S/SH groups
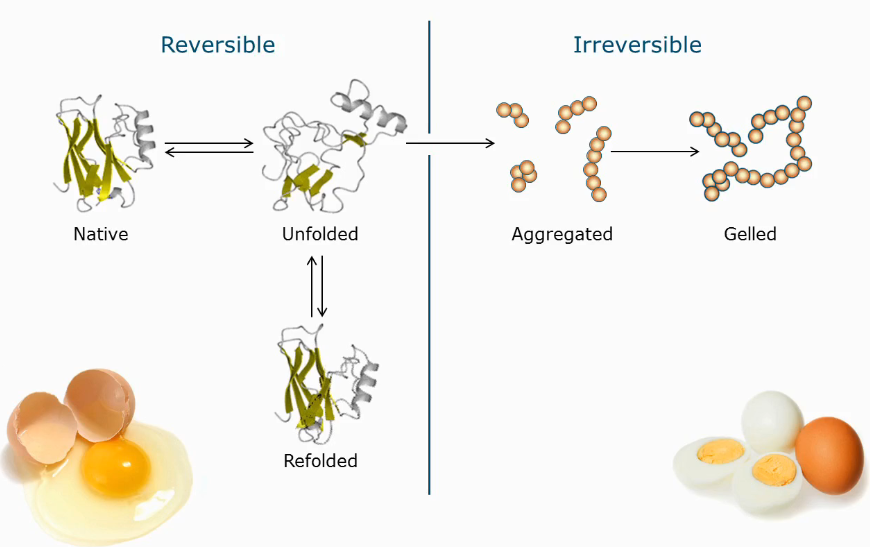
Leads to: unfolding and/or aggregation
When can aggreagtion in proteins also happen if there is no denaturation?
At the iso-electric point
Reversible vs irreversible denaturation/unfolding
Changing the folding of a protein is reversible
Aggregation and gelling is not reversible
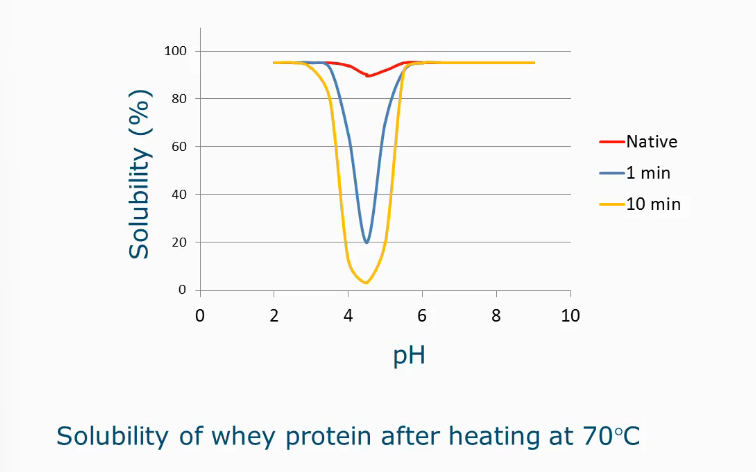
Effect of denaturation on solubility
Aggregation at low and high concentration
Precipitation at low concentration
Gelation at high concentration
Important effects of protein denaturation
Decreased solubility
Aggregation
Increased accessibility of peptide bonds to proteolytic enzymes
Loss of biological activity
Increased reactivity
Aggregation at high ionic strength/low ionic strength
At high ionic strength/close to the isoelectric point, aggregation and gelation is easy
At low ionic strength, or far from the isoelectric point, the electrostatic repulsion prevents aggregation, therefore more proteins will refold.
What reactions cause hydrolysis of proteins that leads to changes in taste:
Enzymatic reaction:
Fermentation products (e.g. cheese)
infant formula/clincal food (for reduced allergenicity)
sports nutrition
Heating (high temperatures/acidic conditions)
animal nutrition (fish meal)
Example of a sweet tasting peptide
Aspartame
Sweetening dipeptide (Asp-Phe-CH3)
Sweetening power is 180 times that of sucrose
The sweet taste is probably related to the polarity of the molecule
Taste of amino acids
The amino acid reacts with the taste receptor on your tongue in three different places.
Binding spot for proton acceptor: carboxylic group
Proton donor: Free amino group
Rest of the receptor interacts with the side chain.
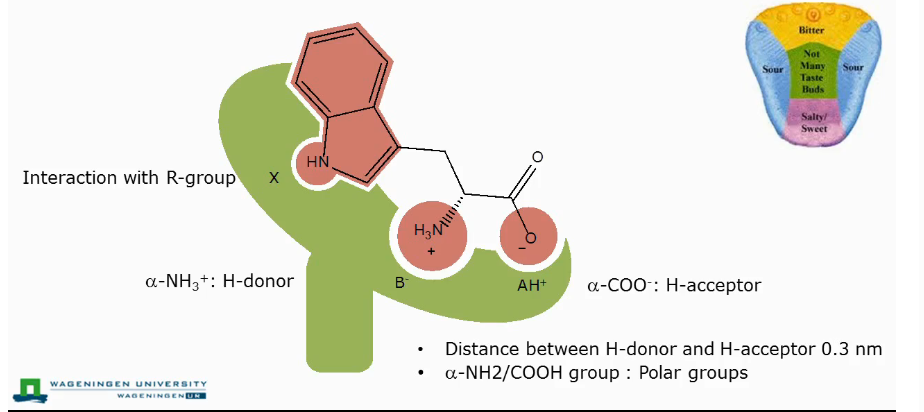
Effect of stereochemistry on taste of amino acids.
This is because receptors are stereoselective
If amino acid has a big apolar side group residue: then we have a bitter taste for L-amino acids but we have a very sweet taste for D-amino acids
In proteins mostly L-amino acids occur and usually are bitter
If side chain is polar or small apolar, the L-amino acid is sweet/neutral and neutral for D-amino acid.
Bitterness of amino acids
Is related to their hydrophobicity
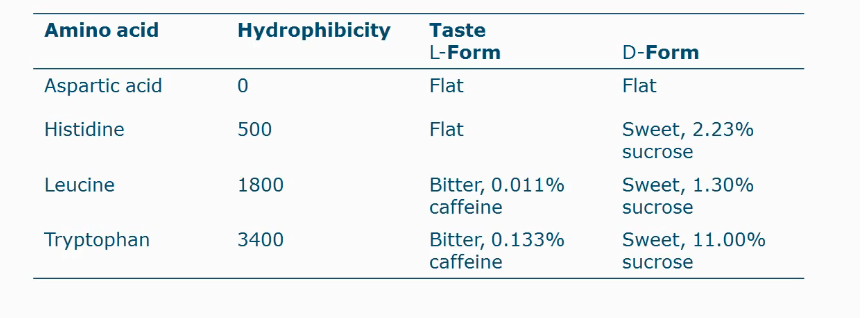
Rules of thumb on taste of peptides
Amino acids in a peptide are more bitter than free amino acids
Non-terminal amino acids are more bitter than terminal amino acids
A peptide is bitter when the average hydrophobicity >1400 cal/mole
Peptides > 6000 Da are not bitter
What does solubility influence?
Visual appearance
Texture (particles in product)
Digestibility
Foam/emulsion properties
Properties determining protein solubility
Balance between hydrophobic attraction and electrostatic repulsion
Charge (depends on pH and ionic strength)
Hydrophobic exposure (depends on folding state)
Presence of polar groups (constant)
Contribution of amino acid side chains ((non)polar,(non)charged)
Non polar non charged: low solubility
Polar non charged: high solubility
Polar charged: high solubility
Where are amino acids usually found on a protein and why + two exceptions
Polar (hydrophilic): predominantly at the surface
Non-polar (hydrophobic): predominantly in the interior
This gives minimum free energy of the system
Exceptions: Cys and Pro
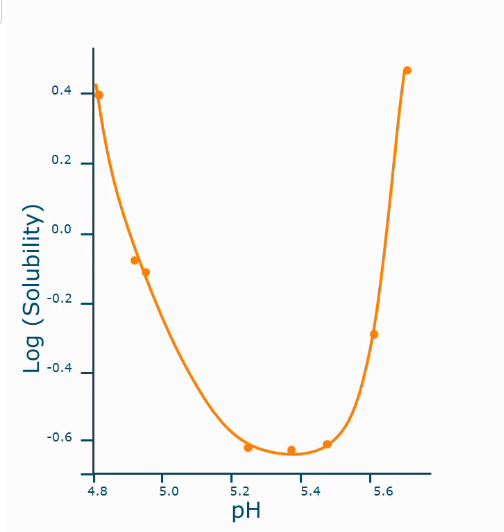
Solubility as a function of pH
At isoelectric point, proteins usually aggregate, and that is usually the minimum solubility level
Solubility as function of ionic strength
The more salt added the more soluble it becomes
Because charges on the protein are neutralized by salt ions
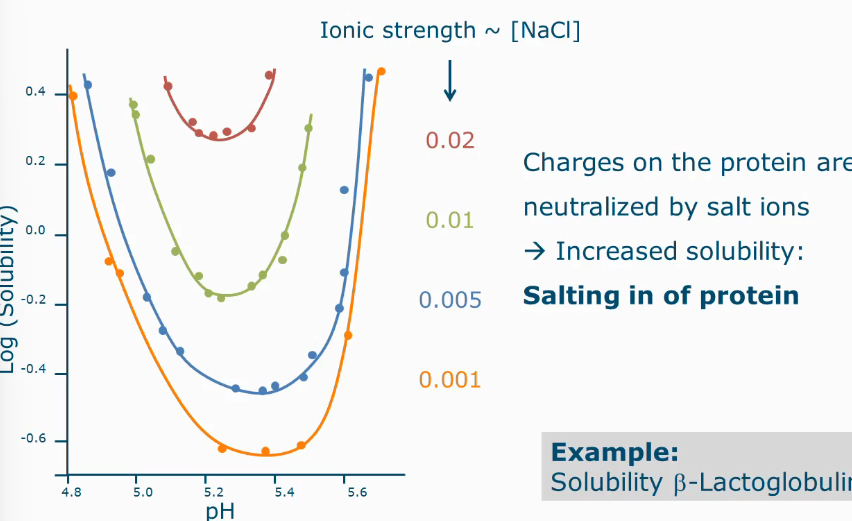
Effect of low salt concentration with protein
There is more interaction of the protein with water molecules
Protein solvent interactions become stronger
Known as salting in of protein
Effect of high salt concentration on protein
Known as salting out
Less water molecules are available to interact with the protein
Protein-protein interactions become stronger than protein-solvent interactions
Analysis technique for (in)soluble proteins
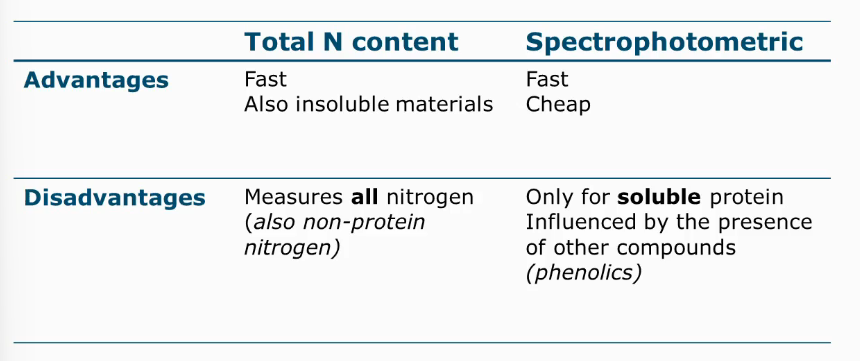
Dumas/Kjedahl methods
Determines N content
Analysis technique for soluble proteins
Direct or indirect
Indirect: proteins in a solution, in addition we put a reagent, a dye.
Dyes: biureet, phenolreagent, coomassie reagent
These dyes form a colored complex in the presence of a protein
More color = more protein
Kjeldahl/Dumas
Determination of total N content:
All amino acids contain nitrogen.
Kjeldahl:
Uses a powder of concentrated sulfuric acid, boil it a 350C
This destroys the protein and liberates the free nitrogen atoms
Dumas:
Total destruction of system, amino acids are burned without acid at 1200C
N gas is released
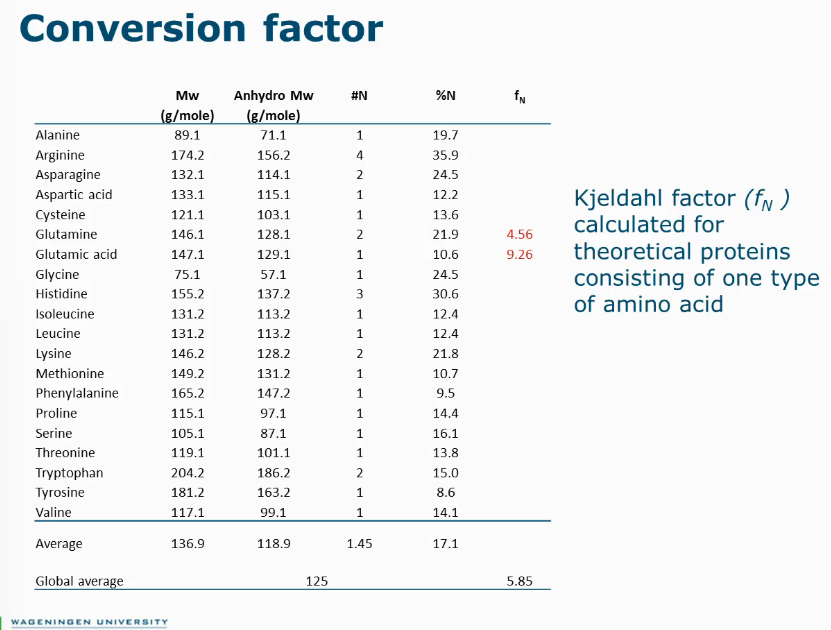
Conversion from N content to protein content
g N/100g DM * conversion factor = g protein/100g DM
Calculate based on amino acid composition
Typically used value: 6.25 (g protein/g N), in most cases this is actually wrong.
Instead, use conversion factor
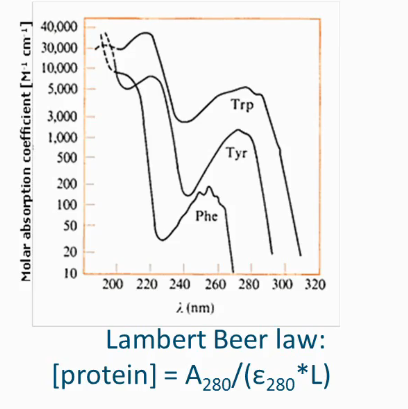
Spectrophotometry
Soluble protein: amino acid with aromatic side chain → conjugated system absorbs UV light
You can use lambert beer law
Advantages and disadvantages of total N content vs spectrophotometric
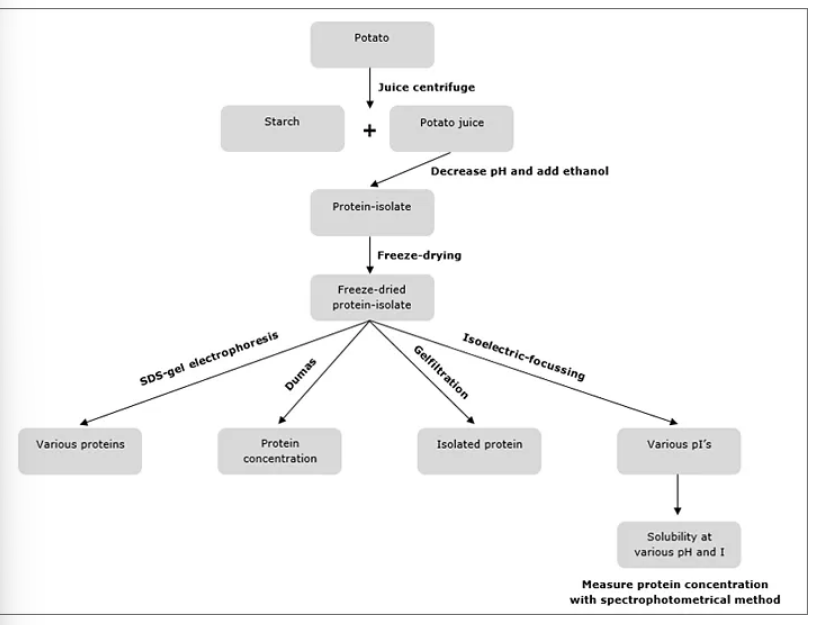
How to find which proteins are present?
SDS polyacrylamide gel electrophoresis (SDS-PAGE):
Unfolding of protein and dissociate complexes
Separation of proteins on a gel under influence of an electric field
Separation distance on gel relates with molecular weight
Calculate molecular weight by comparison with marker (contains protein of known Mw)
All methods used to determine information about proteins