Unit 1 Chem
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
69 Terms
These 8 scientists’ research contributed to Atomic Theory and Quantum Mechanics (in order)
Empedocles
Democritus
Alchemists
John Dalton
J.J Thomson
Ernest Rutherford
James Chadwick
Niels Bohr
Atomic Radius
The distance from the nucleus of an atom to the outermost energy level.
As you go across a period from left to right, this decreases – because the number of protons is increasing, and therefore pulling the electrons closer to the nucleus.
As you move down a group it increases – because additional energy levels are added putting distance between the protons and the outermost electrons. A greater distance means weaker attraction and the atom
expands.
Ionization Energy
The minimum energy required to remove the most loosely bound electrons from an atom
As you go across a period from left to right IE increases - because AR is decreasing electrons are closer to the protons, and electrons are harder to remove.
As you go down a group IE decreases - because AR is increasing, outermost electrons are further from protons and are easier to remove
Ionic Radius
A measure of the size of an ion
Cations are smaller than their neutral atom
they lose electrons and therefore a shell, making them smaller
The protons on the nucleus gain a greater hold on the electrons because their are fewer of them
Anions are larger than their neutral atom
they gain electrons and complete their outmost shell
This means they have more electrons than protons, so the nucleus has a weaker hold on the electrons
gained electrons increase the repulsion among electrons
Electron Affinity
The energy given off of an atom when an electron is gained
As you go across a period from left to right EA increases, because the AR is getting smaller, so the pull from the nucleus is stronger and nearby electrons are easily attached
As you go down a group EA decreases, because AR is increasing, and the attraction of nearby electrons to the protons in the nucleus is weaker
Reactivity
Francium is the most reactive metal - Low IE, lose electrons easily
Fluorine is the most reactive nonmetal - High EA, gain electrons easily
Electronegativity
A measure of an atoms ability to attract electrons within a chemical bond
There is a specific EN for each element
Trend is the same as ionization energy
The smaller the AR the the greater the IE, therefore greater EN
The larger the AR, the lower the IE, therefore lower EN
unitless scale from 0-4
Higher EN, greater attraction of shared electrons
Empedocles Theory
4 elements: Fire, Air, Water, and Earth
All substances are made of a combination of these elements
Democritus
All matter can be divided into smaller and smaller pieces until it is so small it can no longer be divided.
These tiny particles were called “Atomos“ -meaning indivisible
Alchemists
Hired by kings to turn base metals, such as copper and lead, into gold
This was impossible, but they developed glassware and chemical processes that we still use today
John Dalton
After the Alchemists
Developed “Dalton’s Atomic/Particle theory“:
All matter is made up of small particles called atoms that can’t be created or destroyed
Each element is made of its own Atom
Atoms of different elements have different properties
Atoms and 2 or more elements can be combined in constant ratios to form new substances
Dalton’s Model (Solid sphere or bowling ball model)
J.J. Thomson
After Dalton
Discovered the Electron by using Cathode Ray tubes
Plum Pudding model:
Atoms are positively charged spheres
They have negatively charged electrons embedded throughout
Atoms have an overall neutral charge
Ernest Rutherford
After Thomson
Nuclear Atom Theory:
Gold foil experiment:
Atoms are mostly empty space - proven by most light running through
At the centre of every atom is a small positively charged core - the nucleus - proved by how a few particles bounced back from the gold foil
James Chadwick
After Rutherford
Discovered the Neutron:
Neutrons have a similar mass to protons
This explained the different radioactive and mass properties of isotopes
Niels Bohr (State theories, but don’t explain)
After Chadwick
Planetary model
Quantized Energy
Bright line spectrum
Planetary Model
Developed by Niels Bohr
Electrons orbit the nucleus
They exist in specific energy levels of constant energy
When electrons gain energy from heat or electricity, they jump from their ground state to a higher energy level (excited state)
Electrons immediately fall back to ground state, giving off the the energy as coloured light
Quantized Energy
Developed by Niels Bohr
Electrons are in fixed energy levels or shells found around the nucleus
Each level is quantized, meaning they have a specific value of energy
Bright Line Spectrum
Developed by Niels Bohr
A series of bright lines of light produced or emitted by a gas excited by heat or electricity
A light spectrum is used as evidence of a new element, since it it is characteristic to each element
Wave - Particle Duality
This theory explains what keeps atoms from collapsing. (prevents the electrons from being drawn inwards to the protons)
Electromagnetic Radiation
Travels through space as a wave at the speed of light
Contains a range of frequencies known as the EM Spectrum
Photons
Tiny packets of energy that emit EMR
Long wavelength
Low frequency / Low energy
Short wavelength
High frequency / High energy
A quantum of energy
The energy of a photon

Louis de Broglie
He showed that small particles do not behave like large particles, but contain wave like properties, called the Wave- Particle Duality
Erwin Schrodinger
This scientist was after Bohr
He developed wave equations that:
Describe the energy of the electrons
Predict the probability of finding electrons in certain regions of space
Arnold Sommerfeld
He extended Bohr’s work
Proposed that energy levels are divided into sublevels:
S
P
D
F
Werner Heisenberg
This scientist was after Bohr
He theorized the probability model/ uncertainty principles
Can not predict the the exact location of an electron
Can only predict the probability of it being in a certain location
Electrons act more as waves than particles and don’t lose energy
There are 3D regions called Orbitals (not to be confused with Bohr’s energy levels or shells)
Orbital
A 3D space around a nucleus in which Electrons are most likely to be found
Shape represents electron density. not the path they follow
Each Orbital can hold up to 2 electrons
Quantum Numbers
A set of 4 numbers that describe various properties of an orbital
These numbers are like addresses for locating the position of an electron in an atom
The 4 numbers are:
Principle Quantum Number
Angular Momentum Quantum Number
Magnetic Quantum Number
Spin Quantum Number
Principal Quantum Number (n)
This is the first quantum Number
Uses the symbol “n” to label the energy level
The larger the “n“, the larger the size of the electron cloud
1st level n=1 , 1 sublevel
2nd level n=2 , 2 sublevels
3rd level n=3, 3 sublevels
4th level n=4, 4 sublevels
Angular Momentum Number
This is the second quantum number
Uses symbol “I“
Describes additional electron energy sublevels that form part of the main energy level
SPDF sublevels each represent a different shape
S : Spherical - 1 Orbital (2 e-)
P : Dumbell - 3 Orbitals (6 e-)
D : Cloverleaf - or elongated dumbbell with a donut around the middle, 5 Orbitals
F : Complex shapes - 7 Orbitals
Magnetic Quantum Number
This is the third quantum number
Tells us the orientation in space of the electron orbit
Spin Quantum Number
This is the 4th quantum number
represents the clockwise and counterclockwise spin of electrons
Electrons occupying the same orbital must spin in opposite directions
Shown as an up arrow beside a down arrow
Aufbau Principle
This principle states that Electrons fill the lowest energy levels first
Hund’s Rule
This rule states that Electrons remain unpaired as long as possible, meaning the orbitals become half filled before they pair up
Pauli Exclusion Principle
This principle states that there is a maximum of 2 electrons in each orbital, and they must spin in opposite directions
VSEPR Theory
Valence Shell Electron Pair Repulsion Theory
The shape of a molecule is determined by the repulsion between pairs of bonded electrons (like repels like)
Non bonding (lone) pairs of electrons around the central atom have a stronger repulsion of bonded electrons
Polar Bond
Bond contains an EN difference of 0.5 to 1.7
Non Polar Bond
Bond contains an EN difference of < 0.5
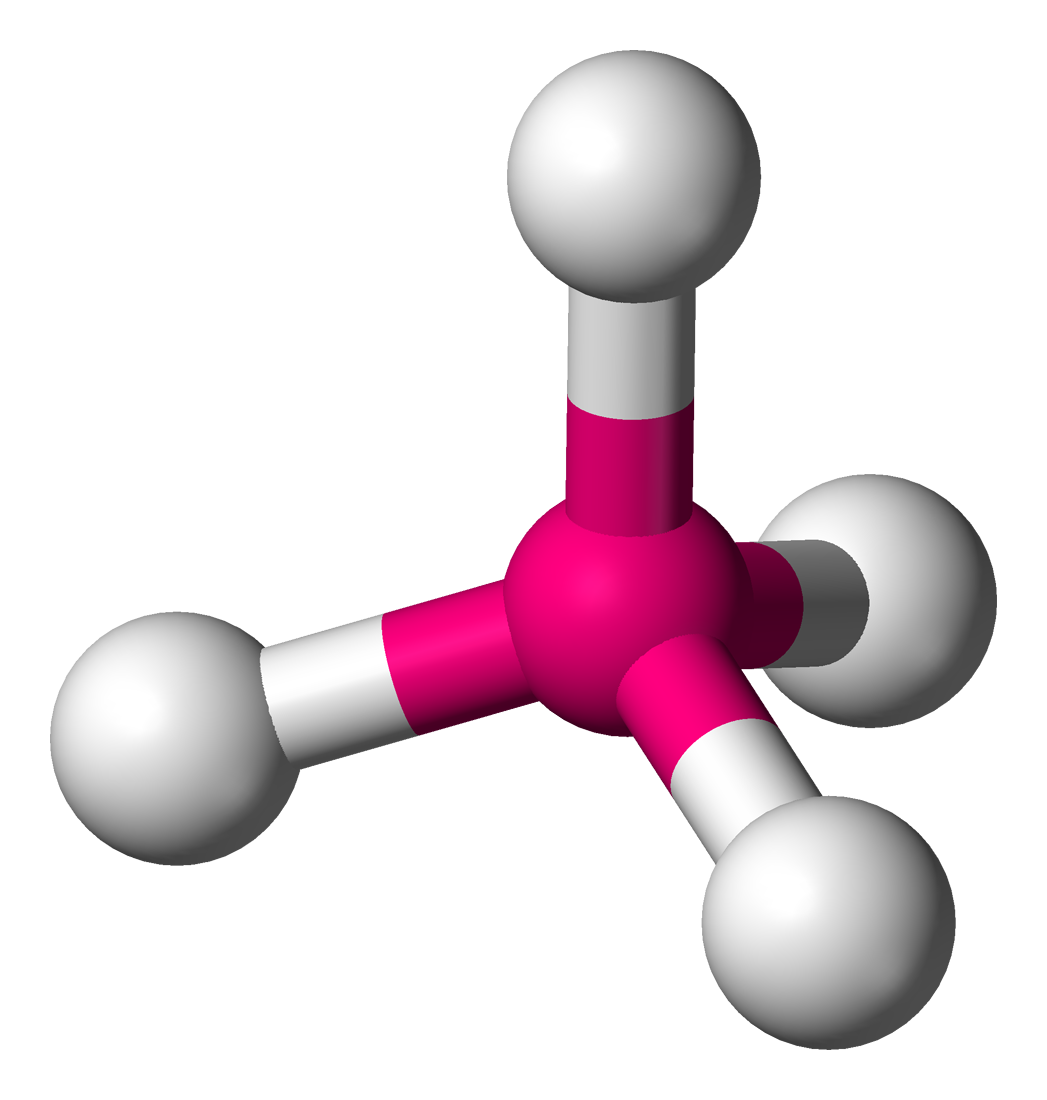
Tetrahedral
Central atom surrounded by 4 bonding atoms and zero non-bonding pairs of electrons (lone pairs)
Bond Angle 109.5 degrees
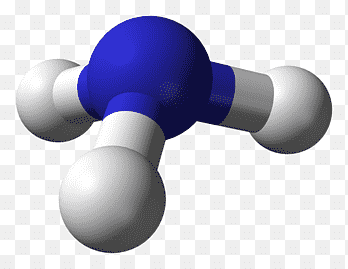
Trigonal Pyramidal
Central atom surrounded by 3 bonding atoms and 1 non-bonding pair of electrons
Bond Angle 107 degrees
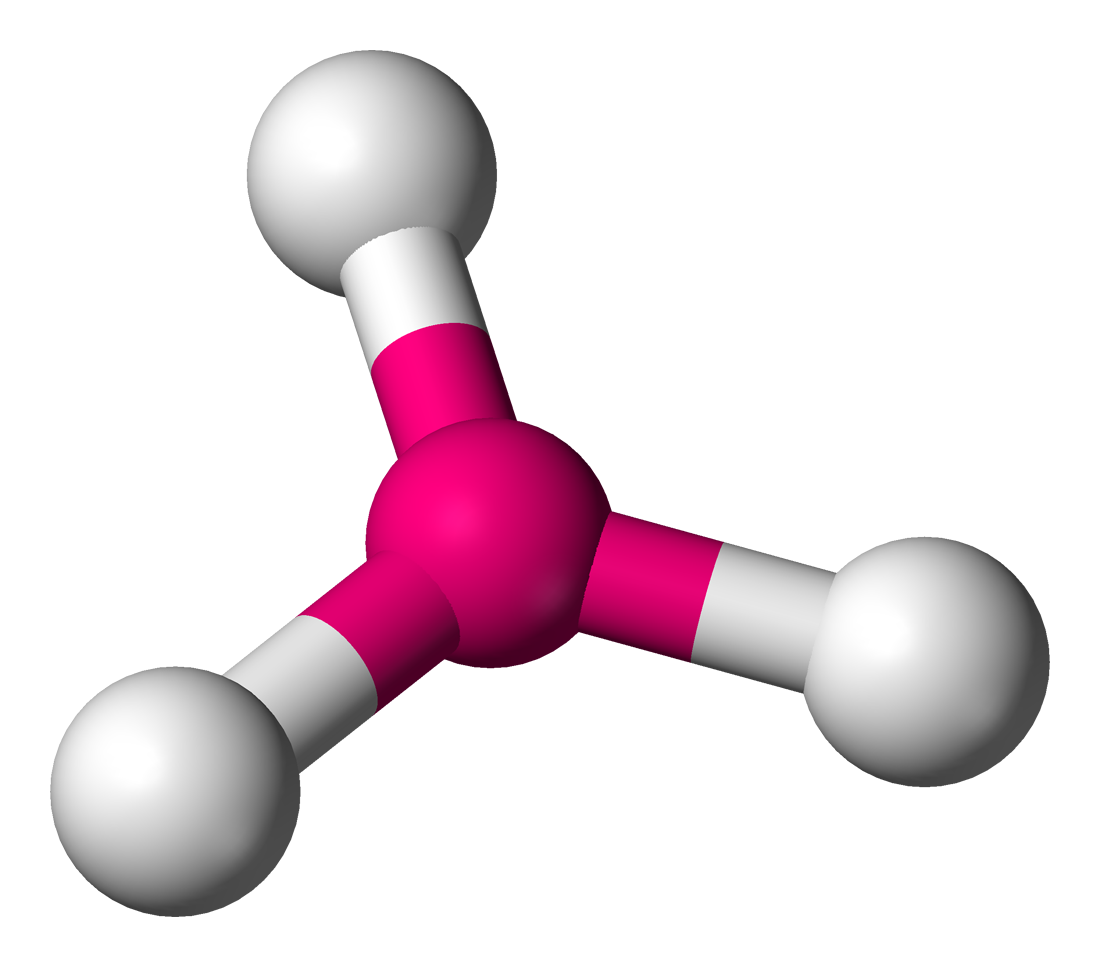
Trigonal Planar
Central atom surrounded by 3 bonding atoms and zero non-bonding pairs of electrons
Bond Angle 120 degrees
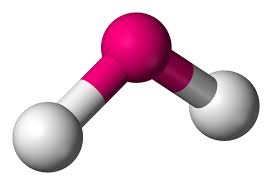
Bent
Central atom surrounded by 2 bonding atoms and 2 OR 1 non bonding pairs of electrons
104.5 degrees
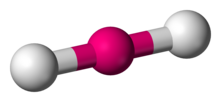
Linear
Central atoms surrounded by 2 bonding atoms and zero non bonding pairs of electrons, OR no central atom
Bond Angle 180 degrees
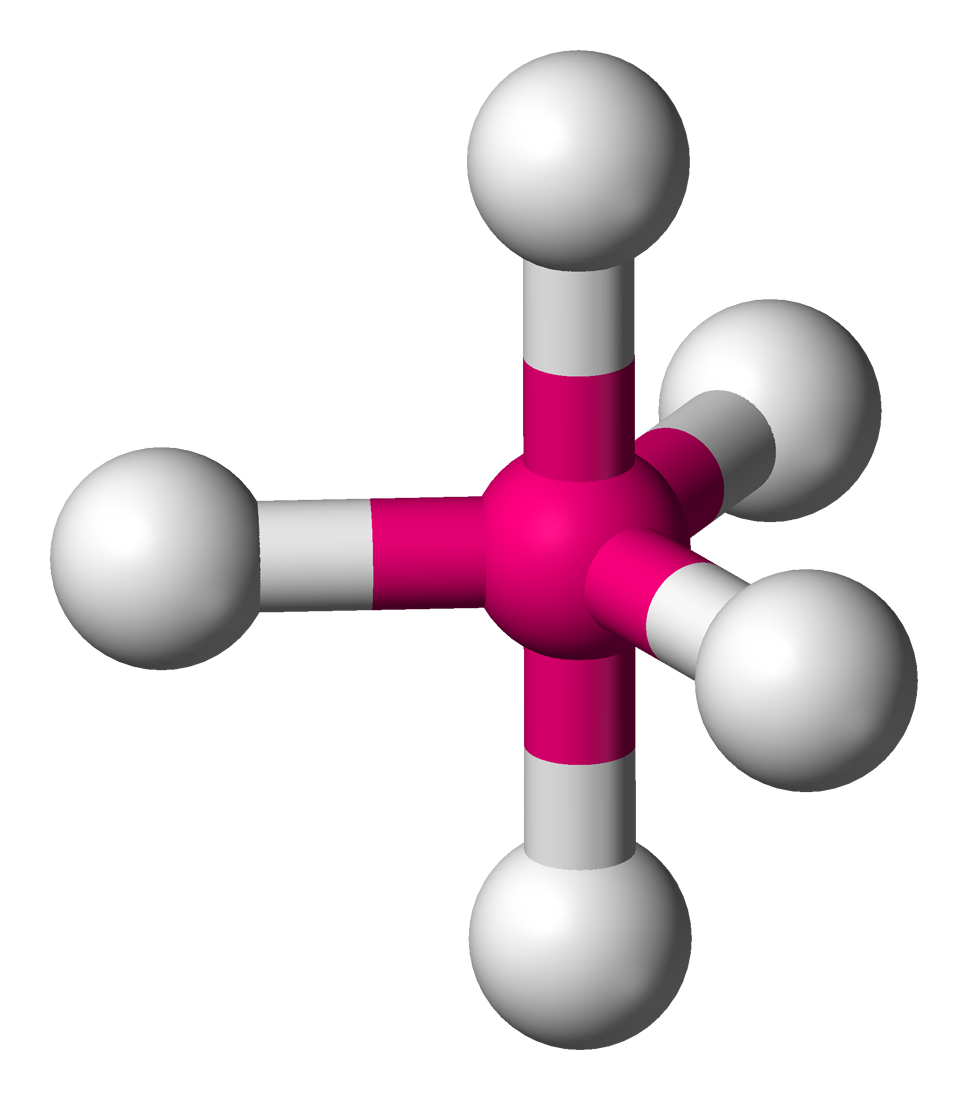
Trigonal Bipyramidal
Central atom surrounded by 5 bonding atoms and zero non-bonding pairs of electrons
Applies to Phosphorus when it bonds to 5 atoms (Octet rule exception)
Bond Angle is 90 and 120 degrees
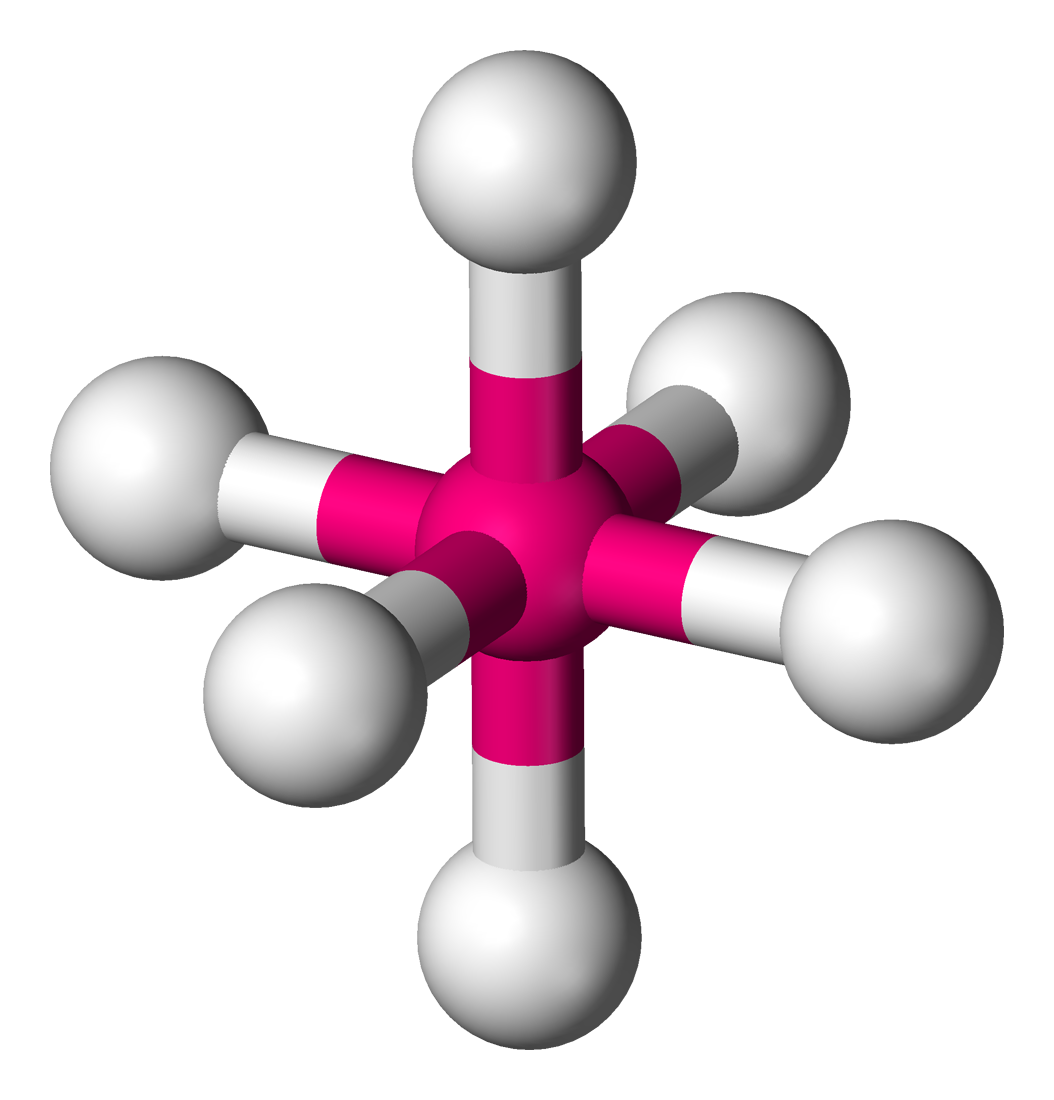
Octahedral
Central atom surrounded by 6 bonding atoms and zero non bonding pairs of electrons
Applies to Sulphur when it bonds to 6 atoms (Octet rule exception)
Bond Angle 90 degrees
Octet Rule Exceptions
Hydrogen: 2 ve-
Boron: 6 ve-
Beryllium: 4 ve-
Phosphorus: 5 bonds = 10 ve-
Sulphur: 6 bonds = 12 ve-
Metallic bonds
This bond is formed from electrons being removed from positively charged ions (metals)
The ions pack closely together, while their valence electrons behave like a mobile sea of electrons
The electrons move throughout the metal
the electrons are called delocalized electrons and they don’t belong to any specific atom
The electrons act as a glue
More electrons = stronger “glue“, higher melting point and hardness
Alloys
Mixture of metals with other metals or sometimes non metals
Alloys are often stronger and harder than the original metal
Added metal may provide extra valence electrons
Different sized alloy metal atoms make it more difficult for metal layers to slip off one another
Two types:
Substitutional
Interstitial
Substitutional Alloys
A type of Alloy
Atoms of added metal can be substituted for those of another if they are approximately the same size
Ex. Brass= Copper + Zinc
Interstitial Alloys
Atoms of added metal are small enough to fit in the space between the other metal atoms
Ex. Steel = Iron (Black) + Carbon (Red)
The 2 groups of Chemical bonding
Crystal Lattices
Small Molecule Interactions
Crystal Lattices and their 3 types
This type of chemical bond has a very large number of atoms bonded together in a regular, repeated sequence, usually 3D.
The three types are:
Ionic
Covalent (Network Solids)
Metallic
Ionic Crystals
This crystal is formed from ionic bonds
There is an attraction between +ve and -ve in the lattice
Metallic Crystals
This crystal is formed from metallic bonds
+ve ions held together by delocalized electrons in the lattice
Covalent Crystals
This crystal is also called a network solid
they are formed from covalent bonds
atoms held together by pairs of shared electrons in lattice
Small Molecules and its two types of forces
This type of Chemical bond contains forces that attract one small molecule to another.
The two types of forces are:
Intramolecular
Intermolecular
Intermolecular Forces and its types
This type of force creates a weaker force of attraction between separate molecules
Weaker than Intramolecular forces
They hold adjacent Covalent molecules together
Exist especially during liquid or solid state
The three types are known as Van der Waal’s Forces:
London Dispersion
Dipole - Dipole
Hydrogen Bonding
London Dispersion force
A Van Der Waal force
The constant vibration of electrons creates temporary dipoles in the molecule
The force of the temp dipoles increases as the molecule increases in size
Caused by a shift in the environment
Found in small molecules
If the charge is produced in one molecule, electrons can be attached
LD is short lived but frequent
Only effective over short distances
Relatively weak bonds
Strength is affected by larger molecules, which have more electrons and more temporary dipoles (becomes stronger)
Dipole - Dipole force
Found in polar molecules with a small permanent dipole
The negative end of one molecule is attracted to the positive end of another molecule
Relatively weak bonds, but stronger than LD
Strength is affected by EN difference in bonding atoms
Hydrogen Bonding
A strong dipole- dipole force between H and O, N, or F of another molecule (High EN difference)
Found in polar molecules with large permanent dipole (F, N, O)
Relatively strong bond
Strength is affected by EN difference in bonding atoms
Strength affected by number of H-N, H-F, and H-O bonds in each molecule
Rank the lattice structures and Van der Waal’s forces from highest to lowest bond strength
Covalent (Intramolecular)
Ionic
Hydrogen (Intermolecular)
Dipole - Dipole (Inter)
London Dispersion (Inter)
Vapour Pressure
The rate at which a liquid evaporates
Stronger forces =
lower rate of evaporation and vapour pressure =
Low boiling point =
High vapour pressure =
Higher boiling point =
Low vapour pressure =
Melting and Boiling Point
These points are measures of the strengths of the bonds/intermolecular forces
It involves the breakdown of the lattice or organized structure between covalent molecules.
Allotropes
Different physical forms of the same element
Ex Carbon
Diamond
3D
Consists of many carbon atoms, such that each central carbon atom is surrounded by 4 others in a tetrahedral