Chapter 9 - Cellular Respiration and Fermentation
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
47 Terms
Fermentation
A partial degradation of sugars or other organic fuels that occurs without the use of oxygen
Aerobic Respiration
Most efficient catabolic pathway, oxygen is consumed as a reactant along with organic fuel.
Anaerobic Respiration
Prokaryotes use substances other than oxygen as reactions, harvest chemical energy without oxygen.
Cellular Respiration Equation
C6H12O6 + 6O2 —> 6CO2 + 6H2O + Energy (ATP + Heat)
Redox Reactions
The transfer of one or more electrons from one reactant to another.
Doesn’t need to involve the complete transfer of electrons from one substance to another, it can be the degree (electronegativity) of electron sharing in covalent bonds.
Redox reactions that move electrons closer to high electronegative atoms release chemical energy.
What products/reactants are oxidized/reduced?
Oxidized: Glucose & Carbon Dioxide
Reduced: Oxygen & Water
Oxidation in cellular respiration
Oxidation of glucose transfers electrons to a lower energy state, freeing energy that becomes available for ATP synthesis.
How is glucose broken down?
In a series of steps to allow it to be harnessed efficiently. If only in one step, the cell cannot handle all that energy at once.
Energy Harvest
Glucose enters the body.
Enzymes called dehydrogenases remove a pair of hydrogen atoms and delivers the 2 electrons and 1 proton to its coenzyme.
Other proton is released as a hydrogen ion to surroundings.
NAD+ , the electron carrier, takes the electrons turning into NADH.
Another electron carrier: FAD, oxidizing agent.
NADH moves electrons to an electron transport chain through various steps.
Each “downhill” carrier has a greater affinity for electrons than the other and is thus capable of accepting electrons from its “uphill” neighbor.
Oxygen catches it at the end, combining it with H+ and forming water.
This process creates energy allowing protons to be pumped across the membrane and thus producing ATP.
Stages of Cellular Respiration & their location
Glycolysis
Occurs in cytosol
Pyruvate Oxidation & Citric Acid Cycle
Occurs in matrix
Oxidative Phosphorylation: Electron transport and chemiosmosis
Occurs in inner membrane.
Substrate-level phosphorylation
An enzyme transfers a phosphate group from a substrate molecule to ADP and produces a small amount of ATP. (Glycolysis & Citric acid cycle)
Glycolysis Summarized
Occurs in cytosol.
Energy investment stage: Cell spends 2 ATP to phosphorylate compounds of glucose and splits into two 3-carbon sugars.
Energy pay off phase: 4 ATP is produced by substrate-level phosphorylation and NAD+ is reduced to NADH by electrons released by the oxidation of glucose. Remaining atoms are rearranged to form two molecules pyruvate.
Occurs whether or not oxygen as is present.
Glycolysis Products
2 pyruvate
2 NADPH
4 ATP (2 ATP used earlier)
2 H2O
Energy Investment Stage
Glucose is phosphorylated by ATP, making it more chemically reactive. The charged phosphate also traps the sugar in the cell.
Glucose is converted to fructose.
Fructose is phosphorylated by ATP.
It is now heavily unstable due to the input of 2 ATP so it breaks into two 3-carbon sugar molecules. (G3P & DHAP)
Energy Payoff Stage
DHAP goes through another process to turn into G3P.
G3P is oxidized by NAD+ and a phosphate group is attaches to the oxidized substrate from the energy released in the oxidation.
The phosphate group is transferred to ADP.
The enzyme relocates the remaining phosphate group.
Enolase (enzyme) causes a double bond to form in the substrate by extracting a water mole, making PEP, high potential energy molecule.
The last phosphate group is transferred to ADP, forming pyruvate.
90% chemical energy from glucose is still unused.
Krebs Cycle
Occurs in the mitochondrion matrix and when oxygen is present.
Acetyl CoA bonds with oxaloacetate, producing citrate.
Citrate is converted to its isomer isocitrate, by the removal of one H2O molecule and addition of another.
Isocitrate is oxidized, reducing NAD+. Loses CO2.
Oxidized and CO2 releases again, remaining molecule is attached to coenzyme A by an unstable bond
CoA is displaced by a phosphate group, which is transferred to GDP, forming GTP. (ATP)
In most animal tissue cells, it produces a GTP molecule by substrate-level phosphorylation. GTP is similar to ATP and may be used to make an ATP molecule or directly power work.
In plants and bacteria cells, it forms an ATP molecule directly by substrate-level phosphorylation.
Two hydrogens are transferred to FAD, forming FADH2.
The enzyme that catalyzes this step is found in the inner mitochondrial membrane.
Addition of water molecule rearranges bonds in substrate.
Oxidized by NAD+ and regenerates oxaloacetate, restarting the cycle.
This is one turn of the cycle, but there are two acetyl CoA from glucose so it is doubled.
Oxidation of Pyruvate to Acetyl CoA
Pyruvate enters the mitochondrion via active transport.
Pyruvate’s carboxyl group [COO-] (somewhat oxidized) is fully oxidized and given off as a molecule of CO2.
The two carbon fragments are oxidized and the electrons transferred to NAD+.
Coenzyme A (CoA), a sulfur-containing compound derived from a B vitamin, is attached via its sulfur atom to the two fragments, forming acetyl CoA.
Mitochondria Structure
Matrix (cytosol), intermembrane space (space between two membranes), cristae (inner folds that increase surface area), double membrane, ribosomes, small circular DNA.
Products of Krebs Cycle
6 NADH, 2 FADH2, 2 ATP, 4 CO2
One turn: 3 NADH, 1 FADH2, 1 ATP, 2 CO2
Electron Transport Chain
Collection of molecules embedded in the inner membrane of the mitochondria.
Components of Electron Transport Chain
Multiprotein complexes numbered I through IV.
Tightly bound to the proteins are prosthetic groups, nonprotein components such as cofactors and coenzymes.
Electron Transport Chain Steps
There’s a drop in free energy as electrons travel down the chain.
Electron carriers alternate between reduced and oxidized states as they accept and then donate electrons.
Electrons from NADH are transferred to flavoprotein.
NADH is less electronegative meaning it willingly gives up electrons.
Flavorprotein passes it down to iron-sulfur protein.
Iron-sulfur protein passes it down to ubiquinone (small hydrophobic molecule.)
The only one that’s not a protein, individually mobile within the membrane.
Remaining electron carriers are proteins called cytochromes, they have an iron atom that accepts and donates electrons.
The last cytochrome passes its electrons to oxygen (oxygen gas) which is the highest electronegative.
Oxygen gas also picks up a pair of hydrogen ions, forming water.
Each compound has a higher affinity for electrons than the previous.
Makes no ATP directly, eases the fall of electrons from food to oxygen, breaking a large free-energy drop into a series of smaller steps that release energy which fuels chemiosmosis.
Establishes the H+ gradient.
FADH2 in electron transport chain
It adds its electrons from within complex 2, at a lower energy level than NADH does.
Provides about one-third less energy for ATP synthesis when the electron donor is FADH2 rather than NADH.
Electrons move from FADH2 to Iron-sulfur protein.
Electrons move to ubiquinone then proceed down normal electron transport chain.
ATP production from oxidative phosphorylation
26-28 ATP
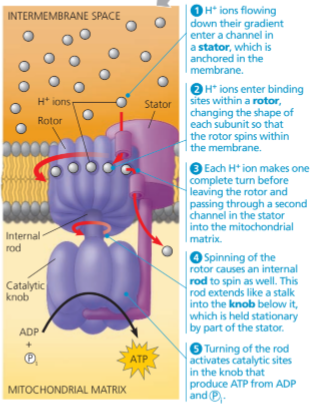
ATP Synthase
Proteins located in the inner mitochondrial membrane, makes ATP from ADP and inorganic phosphate.
Chemiosmosis
Ions (H+) diffuse down their already existing concentration gradient and powers ATP synthesis.
How does electron transport chain create a H+ gradient?
The flow of electrons (electron transport chain) creates energy that is used to pump H+ across the membrane mitochondrial matrix to intermembrane space.
Certain members of the electron transport chain accept and release protons (H+) along with electrons.
Electron carriers are spatially arranged in the inner mitochondrial membrane. They take up H+ and release it.
Proton-motive force
The H+ gradient that results, the capacity of the gradient to do work.
ATP Synthase Process
Total Products Involved in Cellular Respiration
30 to 32 ATP, 10/12 NADH, 2/4 FADH2
Another two FADH can come from glycolysis.
Why is the numbers of ATP produced inexact?
Phosphorylation and the redox reactions are not directly coupled to each other, so the ratio of the number of NADH molecules to the number of ATP molecules is not a whole number.
NADH produces 2.5 ATP.
FADH2 produces 1.5 ATP.
The type of shuttle used to transport electrons from the cytosol into the mitochondrion.
NADH is not permeable so it is either passed to a NAD+ or a FAD in the matrix.
Use of proton-motive force generated by redox reactions of respiration to drive other kinds of work.
Non-shivering thermogenesis
In hibernating mammals or newborns, cellular respiration is reduced to create more heat in a tissue called brown fat (made up of mitochondria).
The inner mitochondrial membrane of brown adipose (fat) tissue contains a channel protein called uncoupling proton (thermogenin) that allows protons to flow back down their concentration gradient without generating ATP, resulting in ongoing oxidation of stored fuel (fats), generating heat without ATP production.
Cellular Respiration chemical energy efficiency
Only 34% of the potential chemical energy in glucose is transferred to ATP, the rest is released as heat.
This helps us maintain our high body temperature, the rest is dissipated through sweating and other cooling mechanisms.
Anaerobic respiration
Instead of oxygen as a final electron acceptor, another molecule is used.
Example: “Sulfate-reducing” marine bacteria use the sulfate ion (SO42-), builds up proton-motive force, but H2S is made as a by-product instead.
Takes place in certain prokaryotic organisms that live in environments without oxygen gas.
Fermentation
An extension of glycolysis that allows continuous generation of ATP by the substrate-level phosphorylation of glycolysis.
Must have a mechanism to recycle NAD+ from NADH.
Example: Alcohol and Lactic Acid Fermentation
Alcohol Fermentation
Used in plant cells, brewing, winemaking, baking.
Yeast and bacteria carry out alcohol fermentation.
Glycolysis takes place (2 ATP produced, 2 NAD+ oxidized).
Releases 2 CO2 from pyruvate, which is converted to a two-carbon called acetaldehyde.
Acetaldehyde is reduced by NADH to ethanol. (NAD+ is regenerated.)
Lactic Acid Fermentation
Used in human cells (oxygen is not reaching the cells in time), cheese, yogurt, acetone, methanol
Fungi and bacteria use lactic acid fermentation.
Some yeasts and bacteria also produce lactic acid from fermentation and produce yogurt and cheese.
Glycolysis takes places, producing 2 ATP and oxidized 2 NAD+.
Produces 2 Pyruvate.
Pyruvate is reduced by NAD+ creating lactae.
Obligate anaerobes
Carry out only fermentation or anaerobic respiration. Cannot survive in the presence of oxygen, can be toxic.
Facultative anaerobes
Can make enough ATP to survive using either fermentation or respiration (yeasts and many bacteria).
Evidence that Glycolysis is ancient
Ancient prokaryotes are thought to have used glycolysis to make ATP long before oxygen was present in Earth’s atmosphere.
Most widespread metabolic pathway among Earth’s organisms.
The pathway does not require membrane-enclosed organelles of the eukaryotic cell, which evolved after prokaryotic cells.
Lactate production in humans
There is a misconception that lactic acid is a substance that makes muscles burn during intense exercise.
Two types of skeletal muscle fibers: Red & White.
Red skeletal muscle fibers: Completely oxidizes glucose to CO2.
White skeletal muscle fibers: Produces lactate from pyruvate even under aerobic conditions, this offers fast but energetically inefficient ATP production.
The lactate product is then mostly oxidized by the red muscle cells in the vicinity, with the remainder exported to liver or kidney cells for glucose formation.
Not considered fermentation.
During exercise, lactate can’t be oxidized to pyruvate. Improves exercise performance.
Real-life examples of fermentation
Decomposition (Bloat stage, H2 and CO2 gases from anaerobic fermentation of gut bacteria)
Plaque and Bacterial fermentation (Lactic Acid → Cavities)
H2 and CO2 gases from anaerobic fermentation of gut bacteria
Cellular Respiration in Prokaryotes
Pyruvate Oxidation takes place in the cytosol.
Citric Acid Cycle takes place in the cytosol.
The electron transport chain is embedded in the plasma membrane.
Oxidative Phosphorylation takes place in the plasma membrane.
Prokaryotes generate H+ gradients across their plasma membranes. They then tap the proton-motive force not only to make ATP inside the cell but also to rotate their flagella and to pump nutrients and waste products across the membrane.
Fats in cellular respiration
Fats are digested to glycerol and fatty acids, the glycerol is converted to glyceraldehyde 3-phosphate, an intermediate of glycolysis.
Beta Oxidation: A metabolic sequence that breaks the fatty acids down to two-carbon fragments, which enter the citric acid cycle as acetyl CoA.
Fats are better fuels due to their chemical structure and high energy level, a gram produces twice as much ATP as a carbohydrate.
Carbohydrates in cellular respiration
Starch is hydrolyzed to glucose, which is broken down in cells by glycolysis and the citric acid cycle.
Glycogen, the polysaccharide that humans and many other animals store in their liver and muscle cells, can be hydrolyzed to glucose between meals as fuel for respiration.
Proteins in cellular respiration
Digested to their constituent amino acids and have their amino groups removed, a process called deamination. Converted to pyruvate, acetyl coA, or citric acid cycle.
Amino acids present in excess are converted by enzymes to intermediates of glycolysis and the citric acid cycle.
The nitrogenous waste is excreted from the animal in the form of ammonia (NH3), urea, or other waste products.
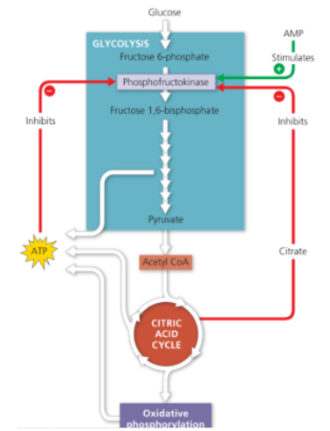
How is cellular respiration regulated?
The cell controls its catabolism. If the cell is working hard and its ATP concentration begins to drop, cellular respiration speeds up. They do this by slowing the rate of certain steps (enzymes) in cellular respiration.
Feedback inhibition is the most common mechanism for control.
Phosphofructokinase (PFK)
Allosteric enzyme that controls rate of glycolysis and citric acid cycle
Inhibited by ATP, citrate
Stimulated by AMP
AMP+ P + P → ATP
Phosphofructosekinase being sensitive to citrate allows the synchronisation of the rates of glycolysis and the citric acid cycle.