Chemistry: Topic 6: The rate and extent of chemical change
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
The rate of a chemical reaction
How fast the reactants are changed into products
Examples of slow reactions
one of the slowest is the rusting of iron
chemical weathering - like acid rain damage to limestone buildings
Examples of moderate speed reactions
metal magnesium reacting with an acid to produce a gentle stream of bubbles
Examples of fast reactions
burning
explosions (faster and release lots of gas). They are over in a fraction of a second
Rate of reaction graphs
The steeper the line on the graph, the faster the rate of reaction. Over time, the line becomes less steep as the reactants are used up
The quickest reactions have the steepest lines and become flat in the least amount of time
What does the rate of chemical reaction depend on?
Collision frequency of reacting particles
The more collision there are, the faster the reaction is
Energy transferred during a collision
Particles have to collide with enough energy for the collision to be successful
Particles need the activation energy to break the bonds and start the reaction
Factors that increase the number of collisions will increase the rate of reaction
Collision theory
Explains how various factors affect rates of reactions
According to this theory, chemical reactions can occur only when:
reacting particles collide with each other
with the right orientation
with sufficient energy
Activation energy
The minimum amount of energy that particles must have to react
Successful collision
A collision that ends in the particles reacting to form products
Factors affecting rate of reaction
Temperature
The concentration of a solution/ the pressure of a gas
Surface area
A catalyst
Catalyst
A substance that speeds up a reaction, without being used up in the reaction itself by providing an alternative reaction pathway with lower activation energy
It is not part of the overall reaction equation
Enzymes
Biological catalysts which speed up reactions in living things
Reaction profile for catalysts
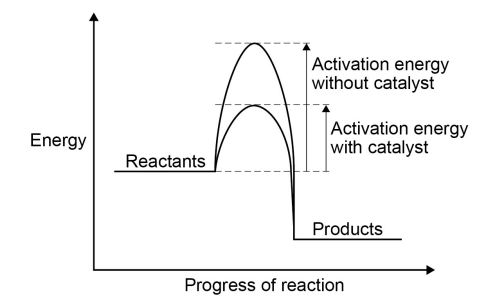
Increasing the temperature increases the rate
When a temperature is increased, the particles all move faster
This means they will collide more frequently
The faster they move, the more kinetic energy they have, so more of the collisions will have the minimum activation energy for the reaction to happen
Increasing the concentration or pressure increases the rate
More concentrated solution means there are more particles the same volume of solvent
When the pressure of a gas is increased, the same number of particles occupies a smaller space
This makes collisions between the particles more frequent
Increasing the surface area increases the rate
If one of the reactants is a solid, then breaking it up into smaller pieces will increase the surface area to volume ratio
This means for the same volume, the particles around it have more area to collide on - so collisions will be more frequent
Using a catalyst increases the rate
Different catalysts are needed for different reactions, but they all work by decreasing the activation energy needed for the reaction to occur
They do this by providing an alternative reaction pathway with lower activation energy
Mean rate of reaction (formula)
= quantity of reactant used / time taken
= quantity of product formed / time taken
Three ways to measure the rate of reaction
1. Precipitation and colour change
2. Change in mass (usually given off)
3. Volume of gas given off
Measuring the rate of reaction - precipitation and colour change
You can record the visual change in a reaction if the initial solution is transparent and the product is a precipitate which clouds the solution (it becomes opaque)
Observe a mark through the solution and measure how long it takes for it to disappear - the faster it disappears, the faster the reaction
If the reactants are coloured and the products are colourless (or vice versa), you can time how long it takes for the solution to lose (or gain it takes) its colour
The results are very subjective - different people may not agree over the exact point when the mark ‘disappears’ or the solution changes colour
You can’t plot a graph from these results
Measuring the rate of reaction - change in mass (usually given off)
Measuring the speed of a reaction that produces a gas can be carried out using a mass balance
As the gas is released, the mass lost is measured on the balance
The quicker the reading on the balance drops, the faster the reaction
You can take measurements of the mass at regular intervals and you can plot a rate of reaction graph
This is the most accurate of the three methods described because the mass balance is very accurate
It has the disadvantage of releasing the gas straight into the room
Measuring the rate of reaction - volume of gas given off
Uses a gas syringe to measure the volume of gas given off
The more gas given off during a given time interval, the faster the reaction
Gas syringes usually give volumes accurate to the nearest cm3, so are quite accurate
You can take measurements at regular intervals and plot a rate of reaction graph using this method
If the reaction is too vigorous, the plunger on the end of the syringe may blow
Calculate the mean reaction rate from a graph
= change in y value / change in x value (time taken usually)
This can be done for a whole graph or between two points in time
The rate finished when the graph goes flat
Calculate the reaction rate from a graph at a certain point
Find the gradient of the curve (slope) at that point
Draw a tangent - touches the curve at one point and doesn’t cross it, then find the gradient of the tangent
Gradient of tangent = change in y / change in x
= 𝚫y / 𝚫x
A reversible reaction
A reversible reaction occurs when the products of a reaction can react backwards to produce the original reactants
When is dynamic equilibrium reached?
In a closed system, when the forward and reverse reactions occur at the same rate and the concentrations of reactants and products remain constant
Factors affecting the position of equilibrium
Temperature - for example: ammonium chloride > (heat) < (cool) ammonia + hydrogen chloride
Pressure (only affects equilibria involving gases)
Concentration of the reactants and products
Describe Le Chatelier’s Principle
If a system is at equilibrium and a change is made to any of the conditions, then the system responds to counteract change and restore the equilibrium
Describe the effect of changing the concentration of reactant and product on the position of the equilibrium
If the concentration of one of the reactants or products is changed, the system is no longer at equilibrium and the concentrations of all the substances will change until equilibrium is reached again
If the concentration of a reactant is increased, more products will be formed until equilibrium is reached again
If the concentration of a product is decreased, more reactants will react until equilibrium is reached again
Describe the effect of changing temperature on the position of the equilibrium
If the temperature of a system at equilibrium is increased:
the relative amount of products at equilibrium increases for an endothermic reaction
the relative amount of products at equilibrium decreases for an exothermic reaction
Describe the effect of changing pressure on the position of the equilibrium
This applies to equilibria that involve gases
An increase in pressure favours the reaction with less molecules
The equilibrium position to shift towards the side with the smaller number of molecules as shown by the symbol equation for that reaction
Pressure has no effect on the reactions where the numbers of gas molecules are equal on both sides of the equation
Describe the effect of a catalyst on the position of the equilibrium
No effect
It just speeds up both forward and backward reactions equally
i.e. equilibrium is achieved faster
Le chatelier’s principle - Changes to temperature example
N2 + 3H2 ⇋ 2NH3
The forward reaction is exothermic - a decrease in temp moves the equilibrium to the right
Le chatelier’s principle - Changes in pressure example
N2 + 3H2 ⇋ 2NH3
There are 4 molecules on the left, but only 2 on the right
If you increase the pressure, the equilibrium shifts to the right
Le chatelier’s principle - Changes in concentration example
N2 + 3H2 ⇋ 2NH3
If more reactants are added (LHS), the forward reaction increases to produce more NH3