Catalysis with and without chymotrypsin
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
What does it look like?
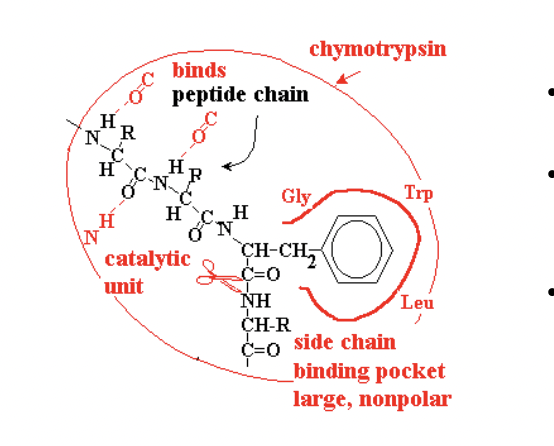
(remember that it binds to residues of trp, tyr, phe) and the binding pocket has these non-polar amino acids that causes favoruable binding to those specific amino acids.
The rest of the peptide will bind into the binding groove of chymotrypsin because of favourable hydrogen bonds
Exaplain chymotrypsin orientationa nd proximity effect
so binding chymotripson its substrate in the way from above is going to position this peptide bond into the right close to the reacting amino acid side chains and in the correct orientation relative to those amino acid side chains for the reaction to occur.
Chymotrypsin binding
it binds weakly to peptide chain upstream of the target amino acids (phe, trp, tyr)
Phe, tyr, trp will fit into the (non polar) binding pocket
if the residues make a good fit, the targeted peptide bond lines up with the caatalytic unit (scissors - the cleaving part)
then we see a strong tight bond
How does chymotrypsin use the proximity effect?
Chymotrypsin binds the polypeptide substrate in its active site, holding it close to catalytic residues (Ser, His, Asp). This increases Z (collision frequency) and allows the reaction to proceed efficiently.
How does chymotrypsin use the orientation effect?
Chymotrypsin positions the peptide bond correctly within the active site so that Serine (Ser) can attack it. This ensures the reactive groups are aligned for efficient catalysis, increasing p (successful reaction probability).
How does chymotrypsin catalyze hydrolysis?
on the c-terminal side of the phe, tyr, or trp it catalyses a hydrolysis of the bond, resulting in two separate peptides.
Because its a hydrolysis we know that its water being added to break those bonds.
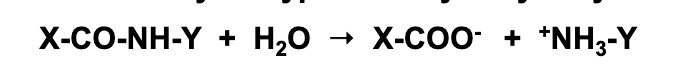
Peptide hydrolysis by H2O without catalysis
step 1)
In the absence of catalyst like chymotrypsin or changing the pH, we just have water.
water can act as a nucleophile (two lone pairs) which can facilitate a nucleophilic attack on the electrophile in the peptide bond.
This attack would be on the carbon thats double bonded to the oxygen
The carbon is electron deficient because the oxygen is very electronegative
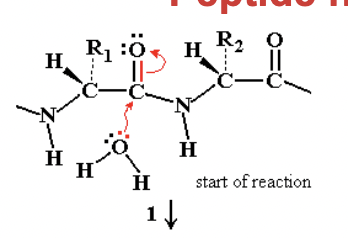
Peptide hydrolysis by H2O without catalysis
step 2)
after nucleophilic attack, we’d get the formation of a new bond to carbon between the carbon and oxygen from the water, which would force carbon to give up a bond.
and the oxygen that was previously double bonded to the carbon is now single bonded and took up some electrons
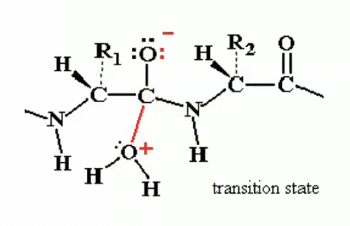
this leads to the oxyanion transition state (sp3 tetrahedral carbon)
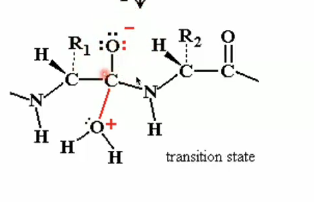
oxyanion
oxygen with negative charge
Peptide hydrolysis by H2O without catalysis
step 3)
transition state may break down ( because its really unstable and really high energy) with Nitrogen as the leaving group
and as seen by arrows the electrons from oxygen would return to carbon to reform that double bond and the electrons from the bond between carbon and nitrogen would go to the nitrogen allowing nitrogen to leave.
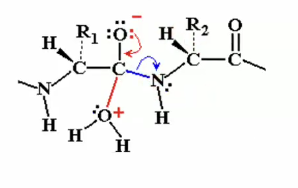
Peptide hydrolysis by H2O without catalysis
step 4)
peptide bond is hydrolyzed and their is an exchange of protons to get NH2
Alternate pathway from transition state without catalyst (step 3B)
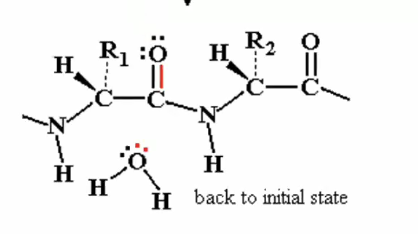
Instead, once the electrons from the oxyanion come back down to the carbon, the carbon, instead of giving up electrons and the the bond to the nitrogen, it can give up the electrons and the bond to the original water. (H20) at the bottom of diagram with the plus)
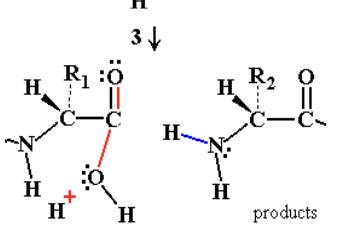
so after the electrons are given to the water we see that it goes back to the initial state, we have the same elctrophile back and no hydrolysis takes place.
What are the possivilities when using water with no cataklyst for hydrolysis?
Both options are likely
we get hydrolysis of the peptide with nitrogen as the leaving group
returning back to original state and water is leaving group (NO NET REACTION HAS OCCURED)
So why use chymotrypsin for hydrolysis and why is it better?
it speeds up the reaction by providing a different reaction pathway (40 reactions per second)
chymotrypsin is going to break this complex water hydrolysis reaction into two much easier steps that arent as likely to end us back up at the beginning.
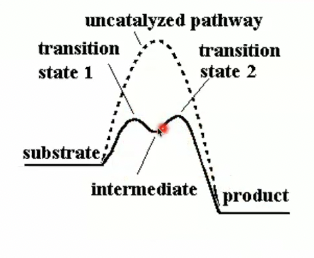
2 Chymotrypsin steps for hydrolysis explained in multiple steps
chymotrypsin will use a nucleophilic group in the enzyme itself to attack the C=O (electrophile)
this will break the peptide bond releasing the C-terminal half of the protein
The N-terminal remains covalently bonded to the enzyme group (acyl enzyme intermediate (represented by the little drop in the diagram.
Water is brought in to release the n-terminal half, and restore the enzyme group to its original state
How does chymotrypsin start the reaction
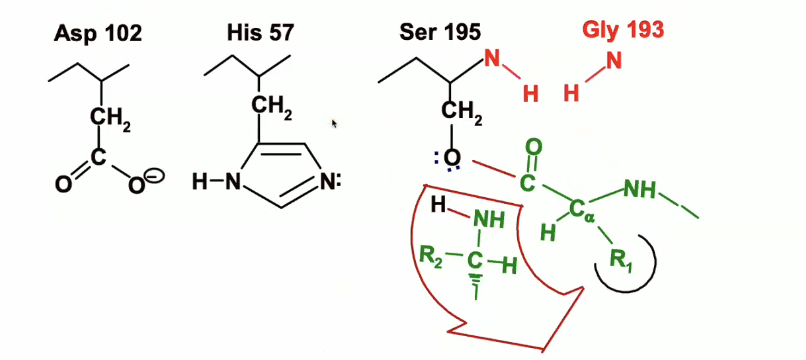
The catalytic triad
Catalytic triad
chymotrypsin uses a better nucleophile in the form of a the catalytic triad, where three amino acids line up side by side and correctly folded in chymotrypsin and cooperate for maximum effectiveness.
the three amino acids are
Aspartate 102
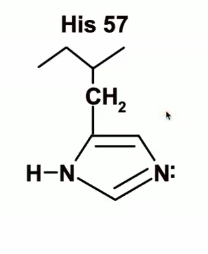
Histidine 57
Serine 195
(numbers indicate position within peptide sequence)
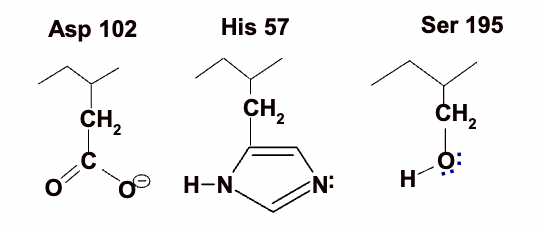
know what its supposed to look like with the lone pairs
Asp 102
negative charge which favours a positively charged partner (like the positive charge on the histidine) which will favour protonated state of histidine 57
His 57
because of the proximity of the negatively charged aspartate will very happily become protonated and become positively charged if it has a proton nearby it can capture
Ser 195
has a hydroxyl group which at neutral pH is protonated but it could give up that proton if theres a lone pair of electrons to share with a suitable atom.
Whats the combined effect of the catalytic triad?
Serine 195 deprotanates and becomes a really good nucleophile.
this is the effect of chymotrypsin decreasing the activation energy
Reminder: how can enzymes decrease the activation energy?
enzyme can change the pathway through the reaction
it can stabalize the transition state
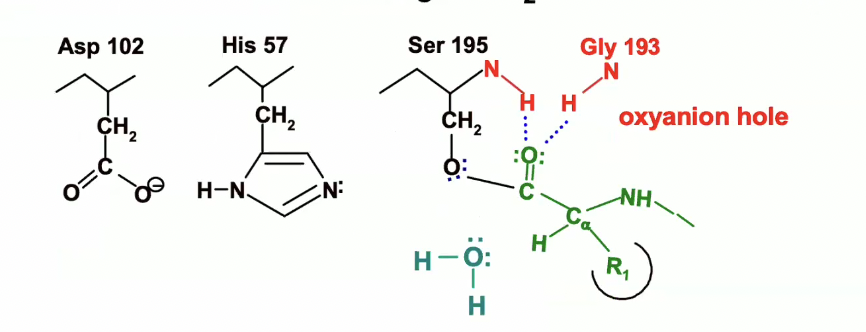
Oxyanion hole in chymotripson
The oxyanion hole is a part of the enzyme's active site that stabilizes the transition state during a reaction.
It contains N-H groups that act as hydrogen bond donors to the negatively charged oxyanion intermediate.
In chymotrypsin, the NH groups of Gly 193 and Ser 195 donate hydrogen bonds to stabilize the transition state.
Stabilization lowers the energy level of the transition state, reducing activation energy (Ea) and speeding up the reaction.
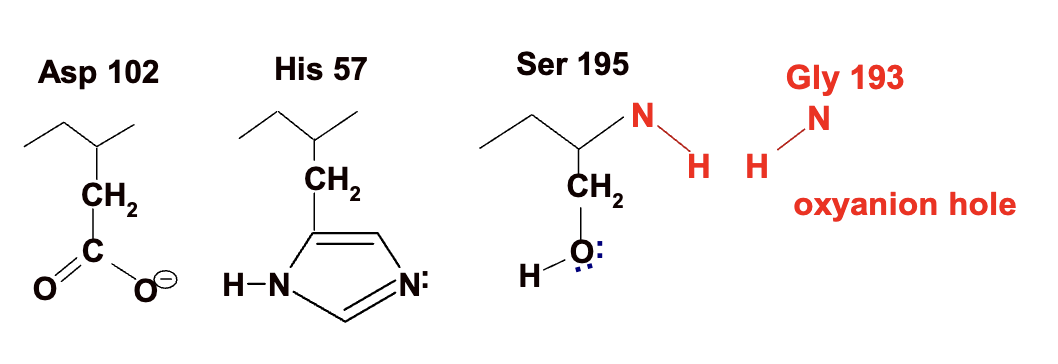
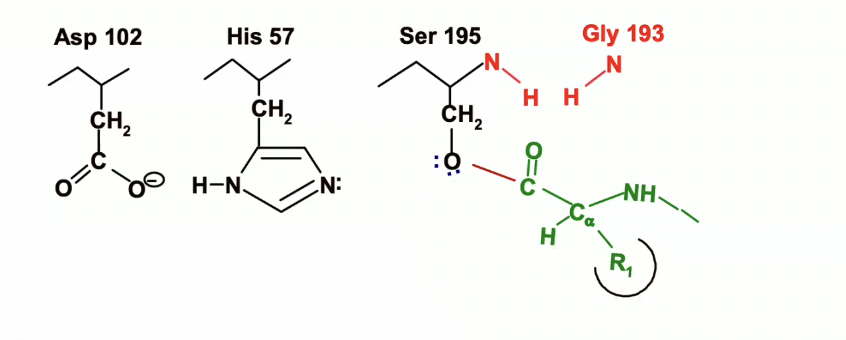
Chymotrpsin hydrolysis steps in detail (step 1) - Nucleophilic attack
This is where the nucleophilic group in chymotrypsin attacks the peptide C=O
the nucleophilic group is the serine in the catalytic triad
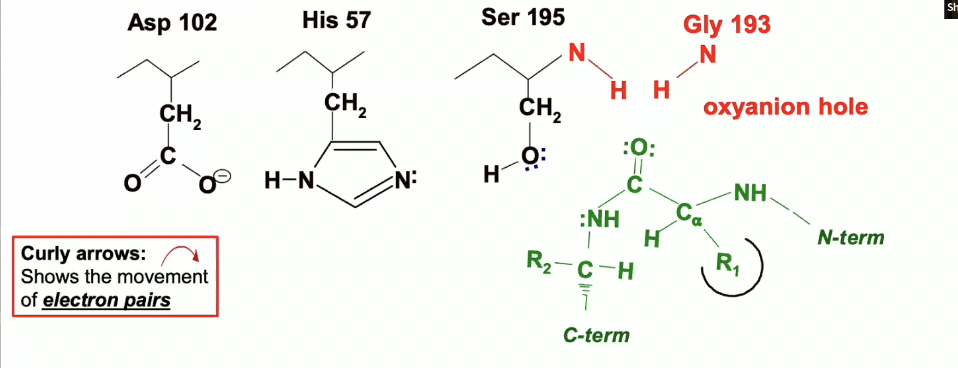
This is the start of the reaction where we see the chymotrypsin active site with the catalytic triad and the oxyanion hole.
in green, we see a peptide/substrate approaching with a trp,tyr, or phe residue (R1) the peptide is specifically located in the binding pocket so that we can position the peptide bond rught near the Serine 195
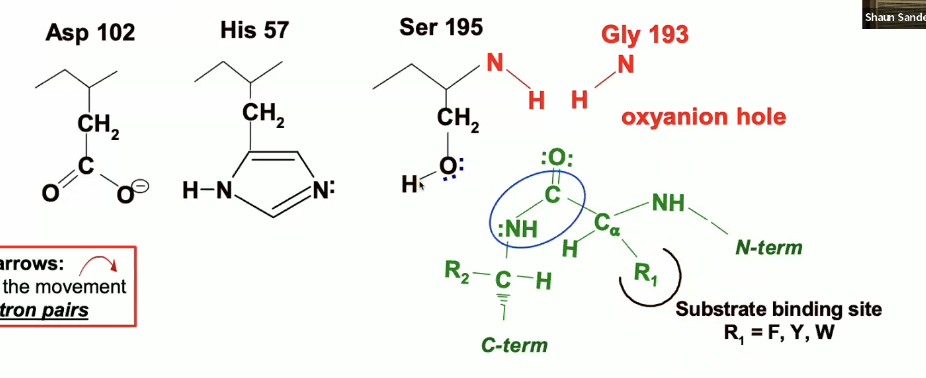
so the reacting groups are that peptide bond in green and the serine 195 which are being positioned very close to each other in the right orientation for a reaction to occur.
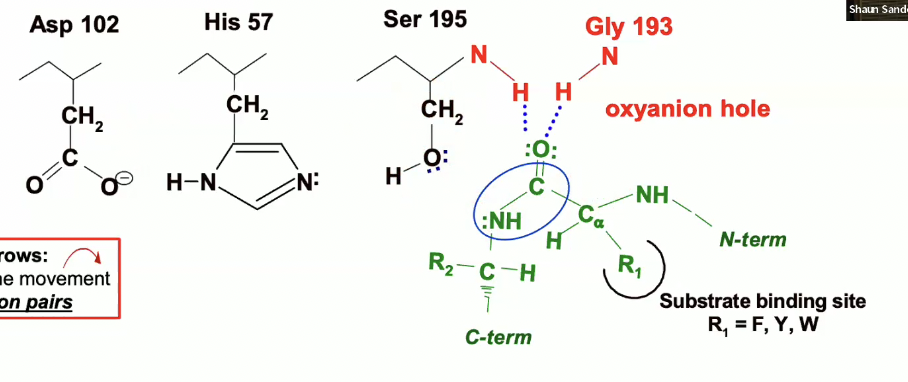
so the oxygen on the carbonyl group (circled in blue) is pointing up towards the oxyanion hole - the blue dotted lines are indicating where H bonds will form (they have not formed yet.
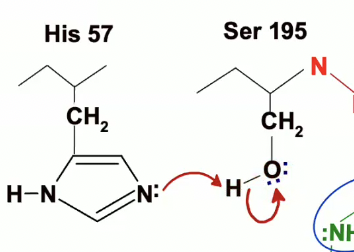
His 57 is going to act as a general base and will remove the proton from OH group on Ser 195 and will take that proton.
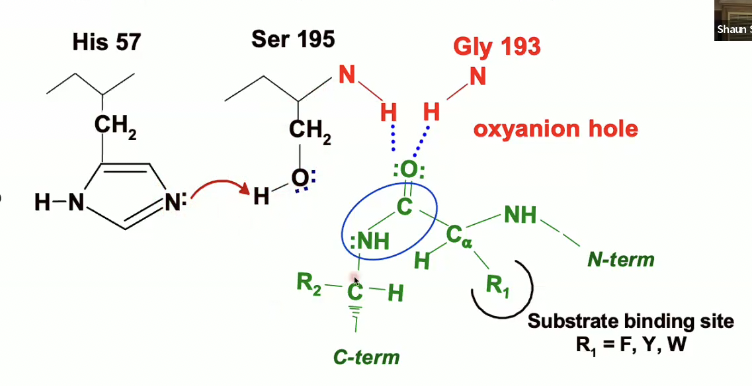
The electrons involved in the bond between Ser 195’s oxygen and hydrogen are going to go the the Oxygen
now Ser 195 is a very good nucleophile, so it will facilitate the nucleophilic attack on the peptide carbonyl group
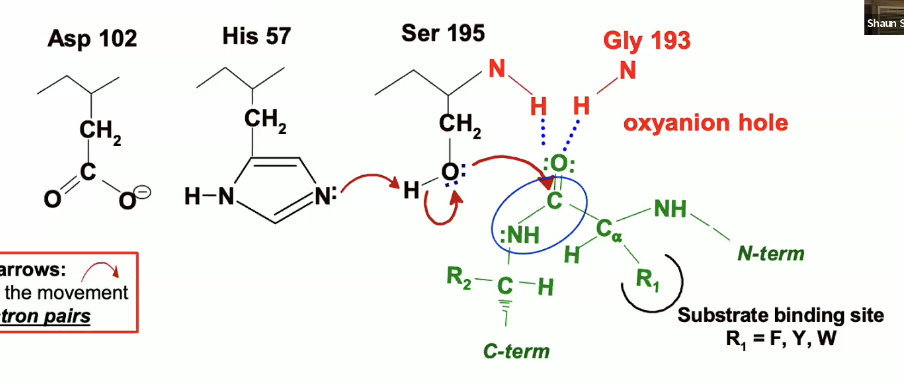
The lone pair of electrons from the serine oxygen will go to the carbon, but carbon cant take any more than 8 valence electrons, so it has to give up a bond, which it does with the double bonded oxygen
the oxygen will take up the electrons from the bond
The negative charge on the Aspartate will delocalize positive charge in His 57, meaning it will take away that charge a bit by pulling it over away from the serine.
Summary of above
Peptide Binding & Orientation
The peptide with an aromatic residue (Trp, Tyr, or Phe) (R1) enters the chymotrypsin active site and is positioned near Serine 195.
The peptide bond is aligned correctly with Ser 195, so the carbonyl carbon is close to Ser 195's nucleophilic -OH group.
Activation of Ser 195
His 57 acts as a general base, removing the proton from Ser 195’s hydroxyl group, turning it into a stronger nucleophile.
Nucleophilic Attack
The oxygen of Ser 195 attacks the carbonyl carbon of the peptide bond, creating a tetrahedral intermediate.
This forces the carbonyl oxygen (circled in blue) to point up toward the oxyanion hole.
Bond Rearrangement
The oxygen of the carbonyl group (on the peptide) forms a new bond with Ser 195’s oxygen.
Electrons from the broken bond will move to the carbonyl oxygen, resulting in a negative charge on the oxygen.
Stabilization
The oxyanion hole stabilizes this intermediate by forming hydrogen bonds (blue dotted lines) to the negatively charged oxygen of the tetrahedral intermediate.
Electron Delocalization
The positive charge on the histidine is delocalized by the Asp 102 in the catalytic triad, enhancing the nucleophilic attack.
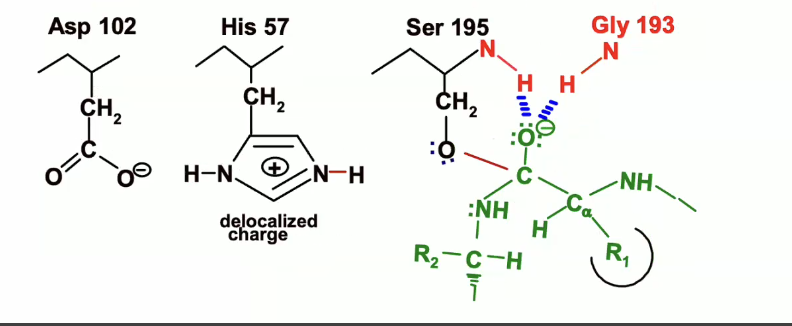
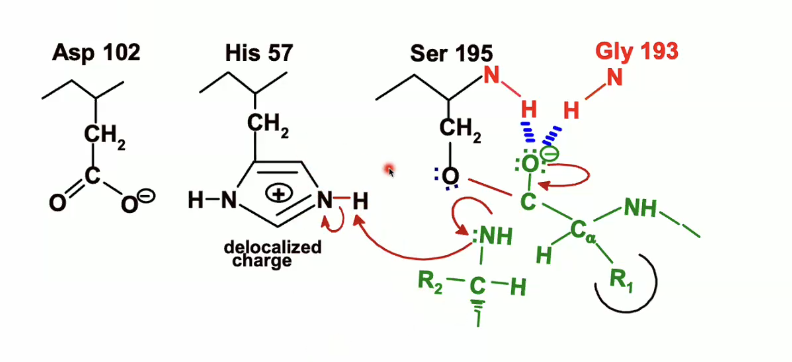
Formation of the first transition state (Chymotrypsin reaction)
Now we have our tetrahedral carbon with the oxyanion on the peptide
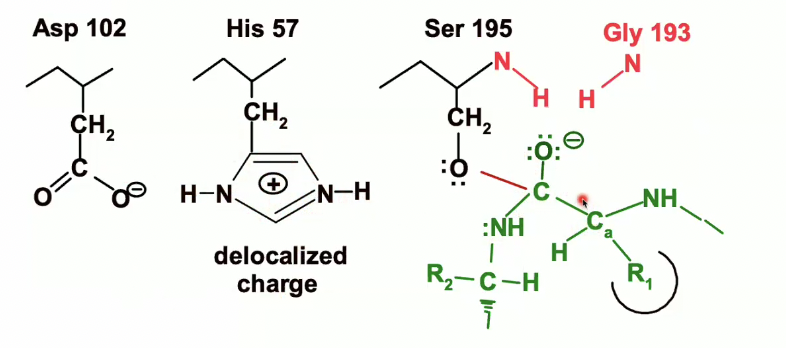
The oxyanion (with the negative charge) will be pulled up into that oxyanion hole and itll form those hydrogen bonds.
This will stabilise the transition state and decrease the activation energy.
When can the hydrogen bonds form between the peptide and the catalytic triad
when the carbon of the peptide is in the tetrahedral carboxyanion configuration.
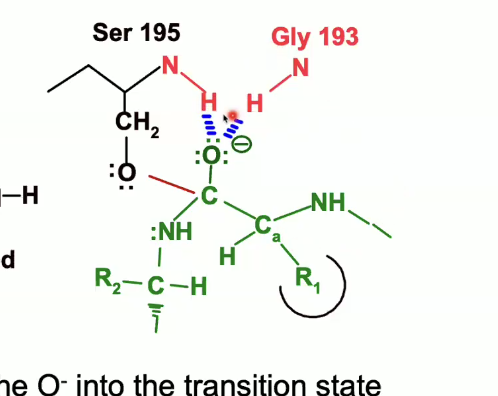
Breakdown of the first transition state (Chymotrypsin reaction)
(remember with chymotrypsin there are two transition states) so to move to the next state we see a breakdown of this first transition state.
Above is what the first transition state looks like. Below is the start of the breakdown.
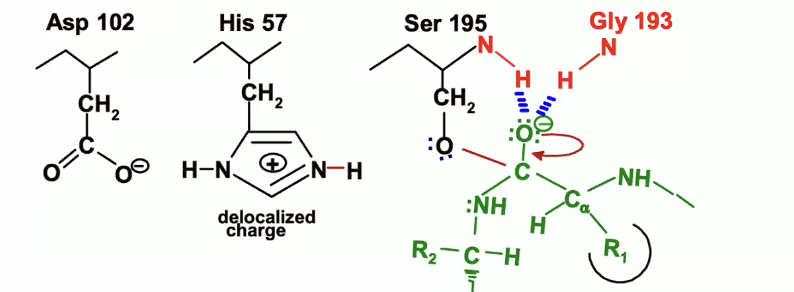
The lone pair of electrons on the oxyanion will come back down and reform that double bond to the carbon
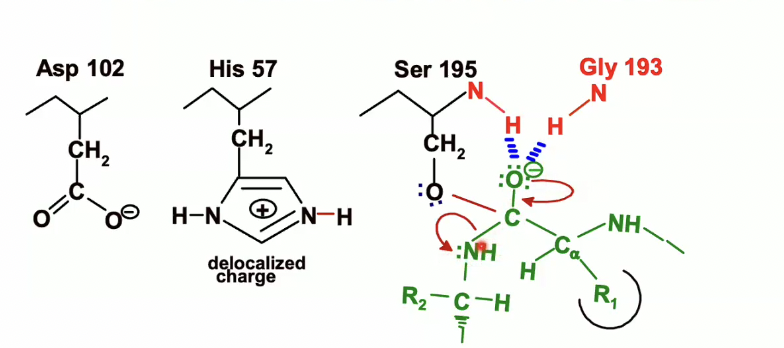
NH bond will act as a leaving group and take up the electrons from its bond with carbon
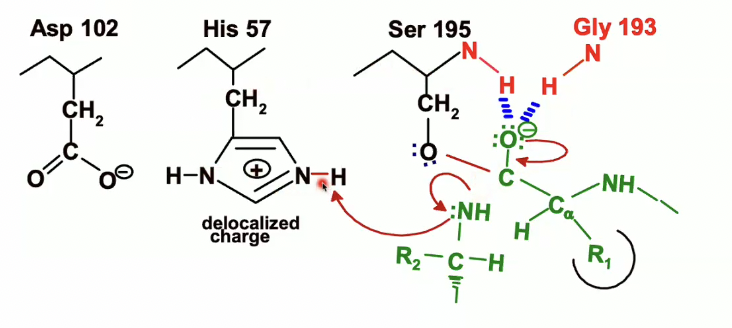
His 57, which was protonated earlier will acted as a general base, will now act as an acid and will donate the proton that it picked up from the serine to the nitrogen of the leaving group
then the electrons from the NH bond on histidine will back on the nitrogen on the histidine which will end up looking like this
Formation of Acyl-Enzyme intermediete
At this point we have a broken peptide bond, regenerated histidine, and the Serine bonded to the rest of the peptide.
We lose the C-terminal half of the substrate, it will leave
we are left with the N-terminal part of the peptide covalently bonded in an ester to the 195 Serine’s oxygen.
This is the acyl-enzyme intermediate (the valley between the two peaks of transition intermedietes)
Chymotrpsin hydrolysis steps in detail (step 2) - Nucleophilic attack
Water enters the catalytic site as seen in turquoise
His-57 will act as a base again which will remove proton from the water
the electrons from the OH bond in the water will go to the oxygen after H is taken up by His 57
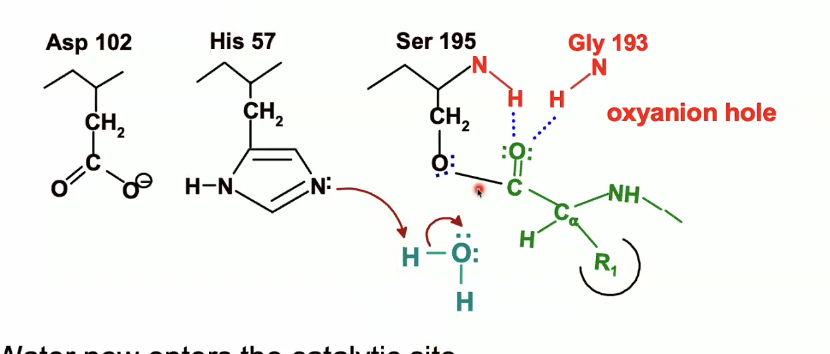
Water now will be an OH^- making it a better nucleophile, which will attack the carbonyl carbon of the peptide.
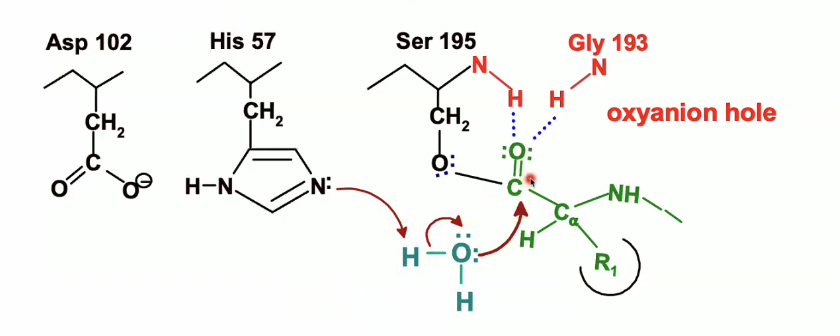
then when that happens, the pair of electrons from the bond with C=O will move to oxygen and Carbon loses its double bond
we end up with the following second transition state
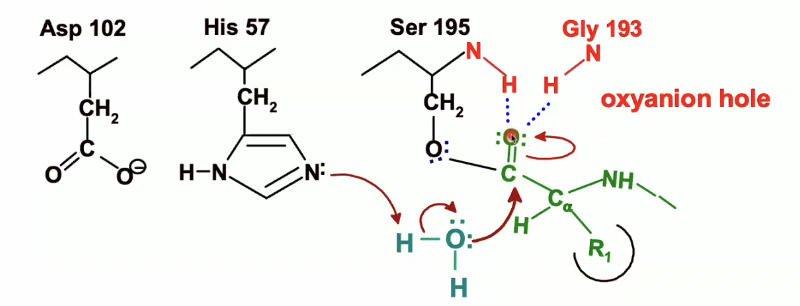
Formation of the second transition state
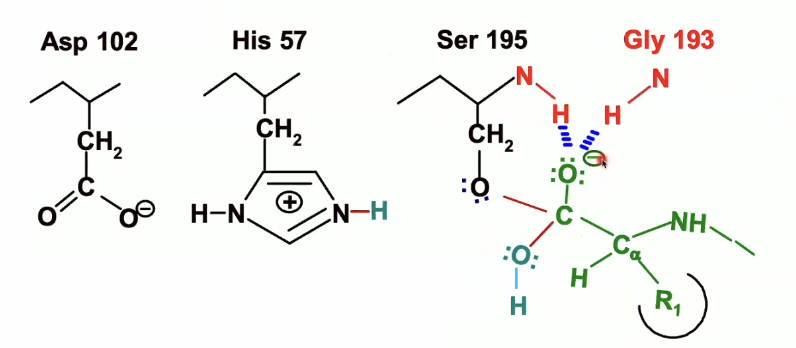
we have that oxyanion now
which will be stabilised by the hydrogen bonds within the oxyanion hole
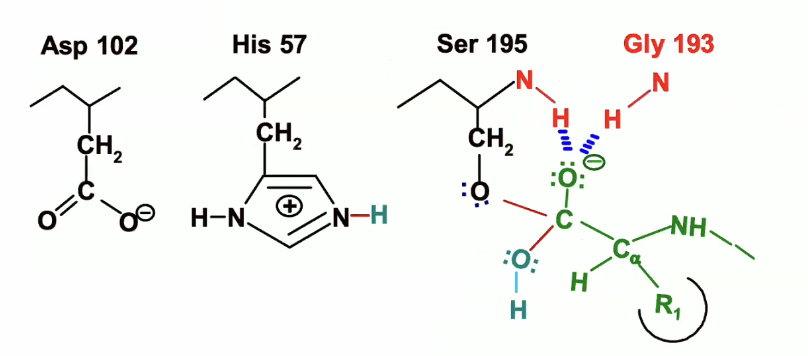
Breakdown of second transition state
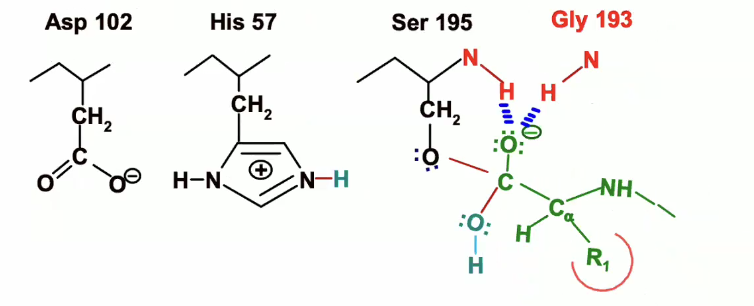
electrons move back down from oxyegn reforming double bond with the carbon
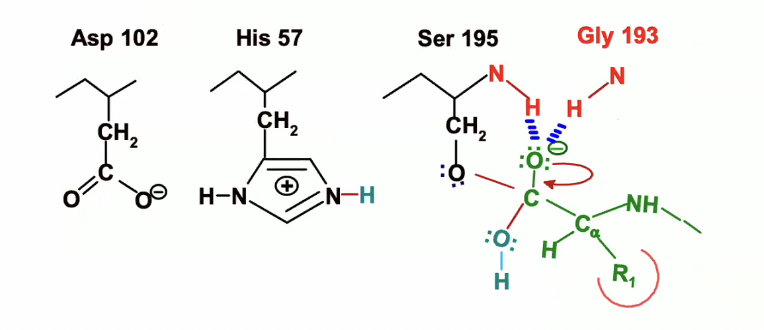
Because carbon can only have 4 bonds we need a leaving gorup and the best one is the oxygen of the serine
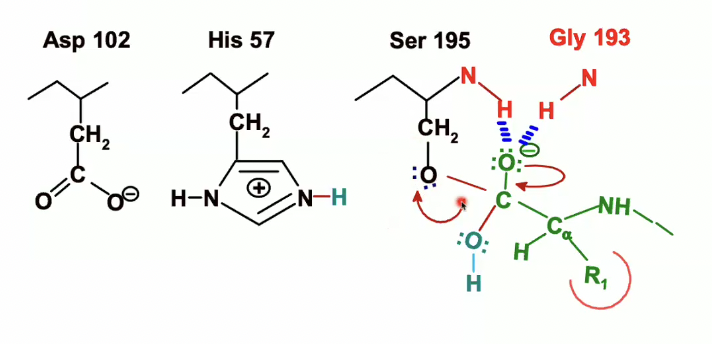
once again, His 57 will act as an acid, donating proton to Ser 195
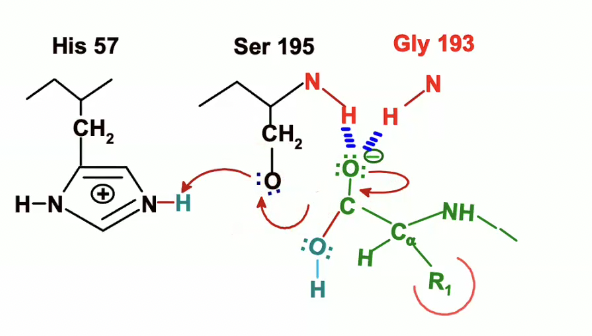
the electrons from the NH will now be given to the N on histidine
The acylenzyme bond is now broken
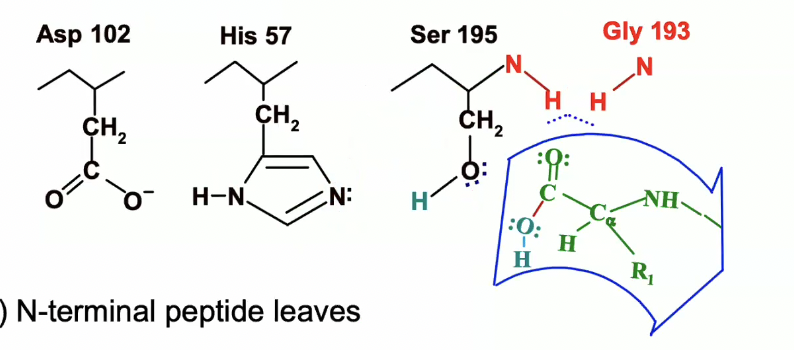
Formation of products
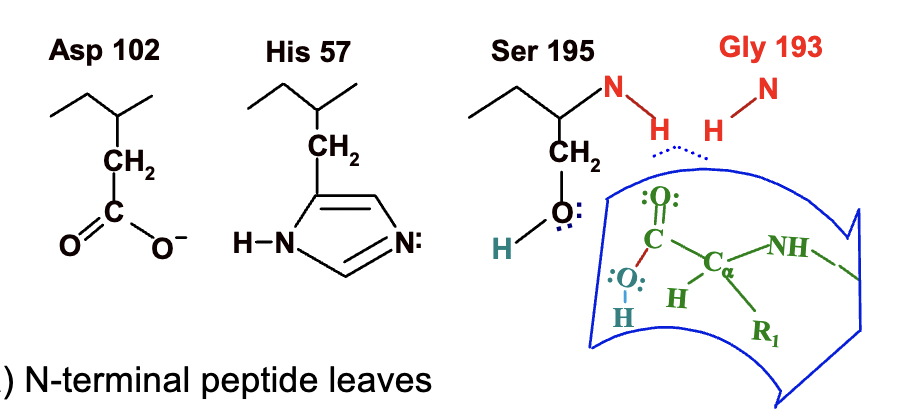
N terminal peptide leaves
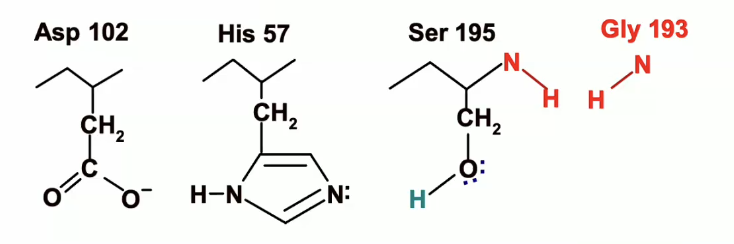
catalytic triad is regenerated (remember thats how it looked in the beginning)
ready to cycle again
What role does His-57 play in the catalytic mechanism of chymotrpsin?
acts as a general acid or general base
His-57 acts as both an acid and a base in the catalytic mechanism of chymotrypsin. How is it able to do so?
because its pKa is closer to the physiological pH.
Chymotrpsin reaction cycle can repeat ___ times per second
40 times/second
How was the chymotrypsin mechanism deduced?
through structural and mutational studies
by replacing Asp with uncharged Ala:
A key finding came from replacing Asp with an uncharged Ala. This mutation led to a dramatic decrease in enzymatic activity, with the enzyme functioning at only 10% of its normal rate. This showed the crucial role of Asp in stabilizing the transition state and facilitating nucleophilic attack during catalysis.
replacing histidine with lysine:
Replacing His with Lys reduces activity to 0.1% of normal, as His (pKa 6.5) acts as both a general base and acid at pH 7, while Lys (pKa 10.2) is a good base at neurtal pH but is a poor acid.
replacing ser with ala: there was pretty much no reaction
Which proteases have identical catalytic mechanisms?
Trypsin and elastase